Jay Fisher - Fine Custom Knives
New to the website? Start Here

"Cassiopeia"
Right now, you are reading the best singular knifemaker's website ever made on our planet. On this website, you will see many hundreds of defined knife terms, detailed descriptions and information on heat treating and cryogenic processing, on handles and blades, on stands and sheaths, and on knife types from hunting and utility to military, counterterrorism, and collection. You can learn about food contact safety and chef's knives, you can find out what bolster or fitting material is best for each application and why. You can lean about caring for a knife, you can see the very largest knife patterns page in history, with many hundreds of actual knife patterns and photos of completed works. You'll also be able to see thousands and thousands of photos of knives, knifemaking, processes, and creations, with many hundreds of pages of appropriate, meaningful text. You might want to know why a knife blade is springy, you might want to know why a hollow grind can last longer than a flat grind. You might want to learn about some pitfalls of the tradecraft, and you might even want to have a chuckle about funny and strange email requests.
You'll find all that here, on JayFisher.com, and you won't find it anywhere else!
There is nothing wrong with cheaper, lower-performance knives, and here you will learn why they are inferior, and what the cheaper knife sacrifices in condition, and thus, function.
It's extremely important to know that the processing of the steel during heat treat is one of the largest successful or detrimental factors in blade steel performance. Along with blade shape and geometry, the knife blade's performance is a result of the knifemaker's understanding of and expertise in steel heat treating process. While people in this field often generalize the relative performance of steels based on anecdotal tales, amateur testing, and popular gossip, most inferior blade steel performance is based on the geometry of the blade and the processing during heat treat. Many steels perform well, and properly processed high alloy steels are the very best steels in the modern world.


"Because tool steels are generally heat treated to make them adaptable to the intended use by enhancing the desirable properties, the behavior of the steel during heat treatment is of prime importance."
--Machinery's Handbook for the Mechanical Engineer, Designer, Manufacturing Engineer, Draftsman, Toolmaker, and Machinist, 1914-Present
Thanks for being here. I created this page as a service to my community of knife enthusiasts, knife collectors, users, aficionados, and knifemakers. I am certain that after you read this page, you will have a greater understanding of modern, high alloy steels used in the finest knife blades, and how steels are physically processed to achieve the very best knife blades ever made in the history of man. We are lucky to be alive in a time when this is possible, and when knowledge and research are available for free—for the advancement of mankind—in an instant.
What I want you to learn from this page is what modern, high alloy, and stainless steels are, what role they play in the world of fine knives, and how heat treating and processing works in my professional field. There is a right way to heat treat knife blades, and it has taken me decades to achieve the level of understanding I have in this field. There is always more to learn, and—God-willing—I'll continue this journey until I'm finished with this world.
Heat treating is the foundation of knifemaking.
I also want you to know what this particular part of knifemaking is not, and what misleading and erroneous ideas are still prevalent, and how inferior and antiquated processes, ideas, and steels are being hyped as of some value other than superficial appearance and tradition. I want my clients purchasing knives because the knives are the best they can possibly be, and that starts with the finest, most advanced metals and treatments that bring them to the pinnacle of their performance.
What kind of performance am I writing about? The performance of knives is cutting, cleanly, repeatedly, and continually. Simple enough; any piece of sharpened metal or other hard material will cut. The performance issue is then about durability, longevity, and strength. These characteristics exist not only in the design of the blade, but also in the steel alloy itself, with advanced metallurgy, scientifically treated, for the highest wear resistance, toughness, strength, and corrosion resistance. This is the working end of the knife, the cutting edge, and performance has to be built into the blade alloy and brought to its most effective physical state by processing, typically done by the knifemaker himself.
"There never was a good knife made of bad steel."
--Benjamin Franklin
A knife is not just appearance, it is first about performance, and that starts with an extremely finely-made advanced technology blade. While the other parts of the knife are just as important, this page deals with heat treating and processing modern, high alloy tool and stainless steels, which far surpass traditional lower carbon, lower alloy blade steels by many orders of magnitude and in many distinctive characteristics.
Welcome to what is perhaps the best page about heat treating modern high alloy custom knife blade steels you will find on the internet, and thanks for taking the time to be here.
When this page went public, some readers (other knifemakers) complained about generalizations on the page: that each statement was not indexed, bookmarked, and referenced. I didn't want to create a thesis-style research document, full of footnotes, endnotes, and references; I wanted an easier-to-read casual text.
If you are a person who doubts what you are reading here, please read every single reference below, and then enter the terms you are doubting into any good search engine (Google is nice) and please do your own research. Then, apply that research to make your own knives.
While I'm not a professional technical writer, I am a professional knifemaker, and doing my best to offer reasonable, specific information on how I do what I do and why I do it. Other knifemakers may post their own research and results on their own sites, backed with their own examples based on their own research and backed with their own successes.
Dear Mr. Fisher,
I just finished reading your new article about heat treating and cryogenic process. WOW, thank you very much for sharing
such lots of information and knowledge. Reading it sure does brings back old memories of college times, as metallurgy is
one thing I studied back in college. The way you describe it amazes me; you do it as like you are a lecturer. Very clear
explanation, so easy to understand.
Thank you for sharing, and keep up the good work.
--Hendrik Rinaldi

"Over 80% of all metals in use are iron and steel alloys"
"Elements of Metallurgy and Engineering Alloys," ASM International, 2008
There is no way to make this simple. Modern media—movies, videogames, and television shows—always tends to show armorers, bladesmiths, and knifemakers heating, forming, quenching, and using blades in highly visual and active procession, with a lot of sparks and fire, and glowing embers scattering around the blade. This may be fine for blades made in the 1800s, but the very best blades are never, ever treated this way. The reality of modern steel and its processing is much more technical. If you just want a brief overview of the process to make superior knife blades, here's a quick step guide:
And that's pretty much it! If you are not interested in the technical nature of blade making, you're done with this page.
That's not really why you're here, is it?
Due to the complexity of the process and material, there is no way—for the sake of brevity—to sum it up with the four steps above. Steel and its crystalline forms are quite complicated, and our understanding of them determines, as knifemakers, knife users, and knife owners the choices and nature of the knife that interests us.
In order to understand the properties of the particular blade, you must first know the steel alloy. There are a vast number of blade steels available in the market today; steel has reached an extremely high level of sophistication and the science will continue to grow. Understanding the nature of this very special material will offer a greater insight into how, why, and where these steels function as they do, and why premium steels are at the forefront of modern technology in nearly every field, not just knifemaking.
This is why it's astounding to discover manufacturers and knifemakers who will not tell you what steel they are using for their knife blades! They offer some undocumented and arbitrarily-assigned name—without disclosing alloy content—when it's clear that the the steel type is absolutely critical to the performance of the alloy and its function, place, and value as a knife! Without demonstrating or revealing even the basic properties of the steel (much less identifying the alloy), the manufacturer or maker of the modern knife is negligent in his service to his customer, or he simply caters to a customer who doesn't care. That's not my client, not my customer, and not my patron.
Another important issue is one of authenticity. Makers and manufacturers are claiming superior blade performance when there is none, and that somehow, a claiming a knife blade made of modern high alloy steel is somehow inferior to the plain carbon steel blade, which is an outright, easily verifiable lie. I do not want to be part of a profession that allows lies to stand for the sake of egos, tradition, or profit, and the best way to eliminate them is with knowledge and scientific facts.
This page is for you—the knife enthusiast, the blade aficionado, and the client, collector, patron, or user who wishes to know why steel is what it is, and how an individual knifemaker can create a superior blade. This page will also make it clear why factory and manufactured knives often cut corners to increase their profit while offering a lower-performance knife overall. There is nothing wrong with cheaper, lower-performance knives, and here you will learn why they are inferior, and what the cheaper knife sacrifices in condition, and thus, function.
Knifemakers will also find this page a useful resource, I'm certain. This page will clarify why modern high alloy tool steels are so special and important to our trade and civilization. It will also clearly show why simple, low alloy carbon steels and hand-forging are crafts based in the romance and antiquated tradition, and high alloy scientifically-processed steel knife blades are the present and future superior performers and premium value.
I'm not here to discard hand-forging, which typically involves lower alloy steels by necessity. If you like a hand-forged or primitive knife, that's a personal preference and I know of thousands of knifemakers who can make this kind of knife for you. The hand-forged knife blade is not the kind of knife I make, for a reason—I've grown beyond 18th-century practices (along with the rest of all modern machinists) for most of my work. I use extremely high alloy hypereutectoid tool steels, and they cannot be hand-forged; they must be treated in specifically controlled processes more like a laboratory than a forge. These are the finest, most state-of-the-art, most advanced tool steels made, and these are the steels I make my knife blades with. More so, these are the steels my clients request, and they are who I make knives for.
This is not a required page for my profession and career—gratefully, I've been successful for decades without having this page on my site. This page is a significant reference and insight into the world of creating effective, superior, and valuable knife blades with some of the finest high alloy steels on our planet. I want to honor you who are reading this with as much viable information as is reasonable, since your time is so very valuable.
The reason I've created this page is because it's my service to the my field, trade, art, and a service to the people who have made it possible for me to do what I do in my career. Improving the performance properties of the highly specialized tool steel blades is critical, and after you finish reading this page, I guarantee you'll know more than most knife owners, most knife manufacturers, and most other knifemakers about this fascinating process.
Thanks for being here!
For complete transparency, please note that since my first knife made in the 1970s, until the present day, I've heat treated every single one to the best of my knowledge and ability. Know, also, that I've never had one failure, not one return, not one complaint about the hardness and wear resistance of a single knife blade I've made.
Heat treating is not mystical wisdom, not a mystery of scientific knowledge, and not an unobtainable goal: it is simply a process. It's hot, it's cold, it's timing, it's workflow. It's numbers, it's temperatures, it's logical, like any process. And like any process, understanding, control, record, and repeatability are the keys for reliable results.
Hi Jay,
Just had to say thanks a ton for all the great info on heat treating and cryogenic treating of knives.
I'm a novice to knives but an analyst by trade, so I appreciate that level of detail to learn more about
the process. The more I learn, the more I appreciate all that goes into the art and science of knifemaking.
--E. B.


Steel is a tremendously important part of our lives. It's everywhere, from the nails and screws that hold our homes together to the vehicles we drive. From the flatware we eat our meals with to the handles of our doors. Steel is part of just about every device, machine, or object that requires some level of durability, and steel is definitely part of the machine or process that allows us to make every device or object we create.
"Steel is the world's most useful and the world's most used metal."
--Robert Raymond
"Out of the Fiery Furnace;
The Impact of Metals on the History of Mankind"
1984
Steel is, then, a creator's dream, and everything that involves steel in any way in our lives is created, because humanity created steel. Steel does not exist in nature; it is entirely man's realm. Though metals may by chance or God's hand in nature come together in rare and random circumstances, steel—as far as we know—came about only because man was tinkering with other metals: copper and tin, and then moved on to iron. Though meteoritic iron was used throughout ancient times, it's a modification of a rock, in essence, and not a direct creation of man.
Iron trinkets were found with bronze works in ancient Egypt, and it is believed that they were accidental creations discovered when smelting copper and casting bronze, evidenced by findings in Israel in ancient copper smelters. Iron oxide (rust and the powdered rock hematite) was used as a flux, a cap to prevent oxidation of copper melt. Some ancient man skimmed off the iron he had used to protect the copper. But then, the iron formed stringy lumps and had to be discarded. Because the melting temperature of iron was so high, they could not do with iron what had been done with copper. For a true melt, the smith needed 1537°C (2800°F), so he had to settle for working the iron in a spongy mass called a "bloom" and by repeated hammering, the slag could be forced out. This is beaten iron, or wrought iron.
About 2500 years ago, an ancient metalsmith created a dagger, with a wrought meteoritic iron blade and a gold hilt (or handle). It's one of the earliest known artifacts made of man-made iron. It was found in the Hattic royal tombs at Alaca Huyuk, near Hattusa in Turkey.
Near Delhi, India, there is a 13,000 pound tower, carefully constructed of pieces of forged iron. The iron is high in phosphorous, so because of this, has formed a passive protective film of oxide on the surface that inhibits corrosion. Uncounted hands have passed by, touching, petting, and stroking the iron and it is polished and oiled by the human tide. It is the largest ancient piece of manmade iron. It was made in about 300 AD, when Constantine established his Capital at Byzantium, when Galerius convinced Diocletian to persecute the Christians, and when the Ostrogoths were subjected by the nomadic Mongols sweeping in from Asia. Manmade iron alloys are very old.
Incidentally, wrought iron is a very specific type of iron, iron with less than .08% of carbon, and it's creation is described above. Other than in conservatory or historic reproduction practice, there is no wrought iron commercially available today! Does that surprise you? It should. What we see sold as wrought iron today is simply mild steel, or low carbon steel. This is the practice of using an old, respected, and traditional name to help sell the romance of the past. What would you think of the architectural railing, table stands, and garden furniture if they sold it as "mild steel, painted black"? No, "wrought iron" sounds so much more… classic.
In the past, wrought iron was not as malleable and formable as cast and forged bronze, so it languished until the Mediterranean basin was conquered by fierce invaders from the sea, whose identity to this day remains mysterious, and the chaos collapsed the entire bronze age. Bronzes disappeared, and more and more, metalworkers turned to iron. Determined to create better iron, the Hittites of Anatolia, peoples of mysterious origin, created a material that was known in their language as "good iron." It was much more durable and superior to wrought iron, and then the Hittites themselves disappeared, prey to European tribes. They left behind the physical evidence of improved iron, and an iron culture that continued widespread.
In the second millennium BC, iron smiths worked furiously with the material and in order to do this, had to expose the iron to white-hot charcoal and carbon monoxide from the combustion. This they repeated over and over to keep the iron hot enough to forge. This exposure forced carbon into the iron, and, simply as a side effect of working with the iron, steel was born. It was harder, stronger, and tougher than iron.
Just .03% increase of carbon in the iron makes into a steel that is harder than bronze. That was the final blow for the bronze age, and the addition of carbon could be directed by exposure in the forge to just the tip of an iron shaft, turning it to case-hardened steel (c. 1200 BC). During that same time, smiths stumbled onto the amazing discovery of the effects of quenching. Maybe they were just tired of waiting for the steel to cool, and wanted to get their projects done, so they quenched it in water. Then a new property was in play.
‘As he finished speaking I handed him the bright wine. Three times I poured
and gave it to him, and three times, foolishly, he drained it. When the wine
had fuddled his wits I tried him with subtle words: “Cyclops, you asked my name,
and I will tell it: give me afterwards a guest gift as you promised. My name is
Nobody. Nobody, my father, mother, and friends call me.”
Those were my words, and this his cruel answer: “Then, my gift is this. I will
eat Nobody last of all his company, and all the others before him”.
As he spoke, he reeled and toppled over on his back, his thick neck twisted to one side,
and all-conquering sleep overpowered him. In his drunken slumber he vomited wine and
pieces of human flesh. Then I thrust the stake into the depth of the ashes to heat it,
and inspired my men with encouraging words, so none would hang back from fear. When
the olivewood stake was glowing hot, and ready to catch fire despite its greenness,
I drew it from the coals, then my men stood round me, and a god breathed courage into
us. They held the sharpened olivewood stake, and thrust it into his eye, while I threw
my weight on the end, and twisted it round and round, as a man bores the timbers of a
ship with a drill that others twirl lower down with a strap held at both ends, and so
keep the drill continuously moving. We took the red-hot stake and twisted it round and
round like that in his eye, and the blood poured out despite the heat. His lids and brows
were scorched by flame from the burning eyeball, and its roots crackled with fire.
As
a great axe or adze causes a vast hissing when the smith dips it in cool water to temper
it, strengthening the iron,
so his eye hissed against the olivewood stake. Then he
screamed, terribly, and the rock echoed. Seized by terror we shrank back, as he wrenched
the stake, wet with blood, from his eye. He flung it away in frenzy, and called to the
Cyclopes, his neighbours who lived in caves on the windy heights. They heard his cry,
and crowding in from every side they stood by the cave mouth and asked what was wrong:
“Polyphemus, what terrible pain is this that makes you call through deathless night, and
wake us? Is a mortal stealing your flocks, or trying to kill you by violence or treachery?”
Out of the cave came mighty Polyphemus’ voice: “Nobody, my friends, is trying to kill me
by violence or treachery.”
--Homer, The Odyssey, Bk IX:360-412
--8th Century BC
The only thing Homer got wrong in his comparison is that quenching is not tempering, at least not during our current times and definition, in this vast history of metals and mankind.


"Steel is not the most popular metal because it's easy to produce and plentiful. Iron is not the most plentiful element and steel is more expensive and complicated to produce. For instance, copper may be found in nearly pure form in many locations on earth. Steel is such an important material because of its tremendous flexibility in metal working and heat treating to produce a wide variety of mechanical, physical, and chemical properties."
--American Society of Metals (ASM), International
Steel, in its basic form, is iron with carbon. Carbon is the number one element in steel that affects its alloy properties. While I'll get deeper into this later, it's important to know that carbon is key. As little as a few hundredths of a percent of carbon in iron makes it steel, and the percentages top out in the standard steel types at about one percent. Remember, I'm mentioning standard steels, not high alloys, tool steels, stainless steels, or specialty steels. I'll start out simply, and we'll get to the really good stuff later!
There are many other alloy elements in modern tool steels, but just for the carbon steel discussion, these are the important and prevalent players.
These are the best steels available today: high alloy steels and stainless steels. Unlike carbon steels, most of them cannot be hand-forged and must be machined (with power tools and by hand) and processed in a clean, scientific, and highly controlled environment. The predominant additional alloy elements in these high performance steels are:
While I could go on and on in the periodic table of elements to detail each alloy, the important thing to know for at this time is steel's relationship with carbon, and how important carbon is. Carbon is the most important alloy in steel and you'll understand why as you continue to read.
This can be a lot to take in; don't bother trying to remember each specific alloy and its contribution. It's enough to know that the relationship of iron, carbon, and the alloy set is synergistic, with the performance of the whole being greater than the individual elements, in strength, hardness, wear resistance, heat resistance, corrosion resistance, and toughness, when properly processed.
Mr. Fisher.
Thank you for your wonderful and well informed site about knives.
So far I have spent quite a few hours reading fascinating info way beyond of what I was looking for.
I have masters degree from mechanical engineering. In the course of my study I have also studied some
[steel] metallurgy subjects. I work as an IT contractor for a large steelmaking corporation. I *very*
much appreciate your very sensible, balanced and pragmatic info on the topic.
I just wanted to say how much I appreciate the info and wonderful advertisements on site - the pictures of your fantastic work.
Best regards
--Stanislav
from Slovakia


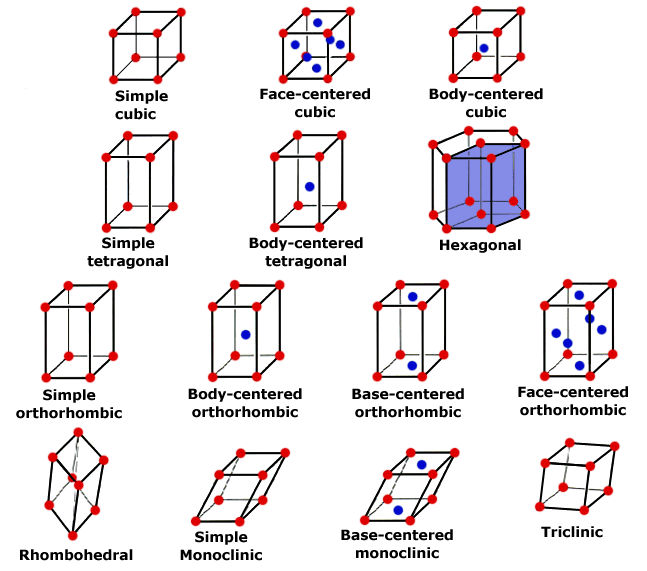
Steel and iron are crystals. This doesn't mean you can hold them up in the sunrise light beams and call the forest nymphs to do your bidding with a chanting spell; it simply means that they have a regular, repeating pattern of atomic arrangement. Like quartz crystals and other mineral, rock, and gem crystals, there is a uniformity of structure based on the bonding of molecules. While there are lots and lots of molecules in, say, a knife blade, for clarity it's best to look at the smallest piece of molecular arrangement, the unit cell. Many unit cells together create a lattice.
It's important to understand that nearly all iron has carbon, but it's not considered steel until the percentage is significant enough to affect phase changes. To get an idea of the variability of iron and steel in context:
From this, you can see that iron and steel have a similar makeup, but they are vastly different materials.
The unit cells, and thus, the lattices of metals are many, but let's start with the simplest, iron. As I mentioned before, it's important to understand that all iron contains some carbon. The carbon atom is only 1/30th the size of the iron atom. Iron only has two unit cell arrangements, face-centered cubic and body-centered cubic.
Referring to the illustration of crystal geometry above. we all know what a cube looks like; it's like a box. In the box of your mind, use a red marker to put a red dot on each corner (8 total). These red dots are atoms of iron. Now add an blue dot in the very center of each flat face (6 total). These are also iron atoms. The face-centered cubic lattice has 14 atoms of iron in this arrangement. This is a unit cell. While it's easy to see in a singular fashion, that cell shares its atoms with the adjacent cells, creating a repeating stack of cells which is the lattice.
The body-centered cubic unit cell has a different arrangement. It's still a cube, but in our box we put one red dot representing iron atoms on each corner (8 total or iron) and one blue dot representing an iron atom in the very center of the inside of the box. So the body-centered cubic unit cell has only nine atoms. Just like the face-centered cell, the unit cell shares its atoms in the corners with adjacent cells in a stack, or repeating arrangement, creating a lattice.
You can visualize these the atoms in the unit cells forming a series of stacked balls. You might consider them visually as ping pong balls, all filling a space, like a bucket. In examining these balls, you'll see that there is space between them. This is where, roughly speaking, many of the carbon atoms reside, in the center of the "holes" between the ping pong balls. Carbon atoms also exist in the interstitial spaces within the unit cells, since the carbon atom is so much smaller than the iron atom.
Those balls touch in relationship to each other, and the arrangement varies. The arrangement is the body packing of the cells, which can be an open or less dense structure of the body-centered arrangement found in alpha-iron or ferrite. The face-centered structure is more dense, and less open structure and it's found in gamma-iron or austenite. More on the phases below.
We humans are are all about heating things. We cook, we bake, we like blowing things up (heating up stuff to a point of massive instantaneous burning or oxidation). Our vehicles combust (burn) fuel, we heat our homes with natural gas (burning) or oil (burning) or electricity (produced by burning fuels). Understanding steel, though, requires a different perspective. We must think of matter and elements cooling or freezing, for that is where the real magic takes place.
When we heat up our iron to become liquid it takes a lot of heat (2790°F/ 1530°C). It looses all of its crystalline structure, just like ice looses its crystalline form when heated to liquid water; everything moves around. It's not the liquefying of the iron that's unusual; it's when it cools, or solidifies into a crystal. As it cools, the atoms lose their energy and bond with nearby atoms to form the crystal lattices. The iron first forms a body-centered unit cell structure and lattice with the the nine atoms. Remember, this is a solid, but it will transform into different crystalline structures while solid. Strangely, as the iron cools to 2550°F/1400°C, the unit cells and lattices change from body-centered to face-centered. Then, in more strangeness, at 1670°F/910°C, the face-centered lattices change again to body-centered unit cells and lattices!
It's fun to visualize this weird structural morphing taking place as the atoms bond, re-bond, move, lose energy, and transform their crystalline form and their molecular arrangement, and the iron and carbon move in form and structure. Remember, all of these crystalline changes happen while the material is solid. The temperatures at which this takes place are critical (important) for us to know, for the next considerations.
The addition of carbon to the iron changes the phases and structure as well as the time, temperature, and characteristics of how the metal reacts and exists. Carbon is introduced into the iron, and occupies the interstitial spaces between the atoms. In essence, the carbon is in a solid solution of the iron. Austenite, ferrite, and iron-carbon alloys are then "interstitial solid solutions."
Mr. Fisher,
Good Morning Sir!
I fully understand that I will not receive a response to this email, however I wanted to thank you for sharing so much expertise,
experience and professional knowledge on your website. It's truly a breath of fresh air to read some no-nonsense facts about
today's knives. The need to run to the newest "whizz-bang ultra premium vapor-deposition" steels these days are almost humorous.
I, too, am a knife maker, although not to your caliber. These days I really only focus on offering various exotic wood handles for knives, and occasion grips for pistols. I always stuck with the tried and true for steels though - 440c, O1, ATS-34 (or RWL-34), 52100, etc.
I only wanted to give you a praise, and thank you for giving a realistic approach in knife making. Keep up the good work Sir!
Most Respectfully Sent,
Kevin S.
USMC (RET.)

It is helpful to understand that when steel cools (or is heated), it undergoes a group of changes in the crystalline structure, forming allotropes of iron. Allotropy is the phenomenon that allows a singular substance to have completely different forms; carbon can be both graphite and diamond. In steels, these changes have been called various things over time, and currently, in materials science, Greek system has been adopted that incorporates some of the older terms to describe these various structures and molecular forms. We also use terms based on the name of the person who discovered or researched the phase (austenite and martensite), and we also have names based on the appearance of the phase structure when viewed through a microscope (pearlite). We even name some of the structures for their physical properties (cementite). This confusing mish-mash of terms comes from many directions, from discovery, industry, research, and applications of steel in our modern world. It's important to know what these terms are, and how they interrelate, because steel metallurgy science crosses so many disciplines.
Please understand that steel and transformations that occur are well known, but there are also some things that are not understood! Though we trust our current knowledge of steels (and other metals) we still have a lot to learn, and that's exciting! Here are the terms:
It's okay if you don't completely understand the term descriptions below; there is a lot to absorb. Steel phases and transformations can be quite detailed and complicated. Some of the descriptions are repetitive, since these phase structures all interrelate and are formed in interactions with each other.
Picturing the geometry of the crystalline structures of unit cells and how they stack, repeat, and are arranged is a textbook in itself. If you are interested in these structures, there are some great reference books identified below. Courses on metallurgy are a great educational tool, if you have the time and money.
The short version in our particular material—steel or Fe-C (iron with carbon)—is this: there are definite geometric spaces created within the structure of a unit cell of both austenite and ferrite. These spaces are identified as either octahedral or tetrahedral. This is a result of the arrangement of the iron atoms.
In these spaces, the carbon in the solution can be accommodated. These spaces are defined as interstitial voids. The voids in austenite are at a different location than in ferrite. These voids are larger than the voids in ferrite. Also, in austenite, the crystalline lattice expands somewhat to accommodate the carbon. In ferrite, the voids are much smaller. The tetrahedral interstices are also not symmetrical as in austenite, and a carbon atom there would displace the iron atoms, so the carbon tends to locate in the octahedral interstices. This means that ferrite will accommodate much less carbon than austenite.
This may seem a bit counter to logic. A unit cell structure that has 14 iron atoms has more available space for carbon in it that a unit cell structure containing 9 iron atoms. You would think that less iron atoms would allow more space for the carbon, but it is not the case because of the octahedral and tetrahedral interstices and their arrangement and locations.
An extremely simplistic way of perceiving this is with balls, often used in molecular and crystalline arrangement illustration. If you stack balls in neat, straight and axis-aligned arrangement, there are large gaps between them. Stacking them this way represents a face-centered structure, with five balls forming a side, there is a lot of room in the "cube." This represents austenite.
If you allow balls to simply locate themselves in a container, they will not stack; they will be offset between the layers, and less space will exist between them. There will be less room between them in the "cube." This represents ferrite.
I hope this simple idea helps. Please don't attack me for my generalization; I'm trying to keep it simple, and I know it's not! So, simply put, austenite accommodates more carbon atoms in solution than ferrite.
The predominate allotropes, constituents, and crystalline structures for our specific discussion of fine knife blade steels are :
Subject: Thank you for your written material
Hello Jay,
I am impressed by your material published and your will to leave behind not only
your good knives, but also your knowledge and long experience into a very narrow field.
As you do not expect, I am a Senior Design Engineer. We are manufacturing and designing quality triggers, mainly for hunting guns. Currently I am learning a lot from your written knowledge experience of using 440C stainless steel.
In some of our applications, we are using this material from solid or sintered. There are some properties discrepancies from batch to batch. We are interested in toughness, superficial hardness in a limited area and corrosion resistance. There are variations from part to part instead to have constant properties. After I will collect all data regarding our manufacturing process, in case I will not be able to find a conclusion and a way of improvement, then I will ask for your opinion.
Anyway, your material about Heat Treating and Cryogenic Processing of Knife Blade Steels: Crystal Unit Cells and Internal Interstice Geometry is very valuable for my work task.
Thank you and Best Regards,
Florin Hristu,
P. Eng.
My Response:
Hello, Florin.
Thank you for your very kind email. I’m honored that you appreciate my work.
Florin, I have seen this before, and after working with 440C for over 40 years, have been able to achieve process and methods to eliminate any irregularities. Because of the outstanding properties of this steel, it is, by far, my most requested blade steel for high corrosion-resistance, wear resistance, and toughness.
I’ll be very interested in what you discover, and if you wish, available for professional consultation.
Thank you again,
Jay
Hello Mr. Fisher,
As the title of this email already says, each time I am visiting your website (daily :) ) I become even more and more impressed.
You are for sure the best knifemaker alive and not only for your gorgeous work but also for your vast knowledge.
Any visitor, no matter of his profession will definitely find in your website a reason to go further, to learn more and to
improve reaching for perfection. I never tried to find a fault in your work as I am sure it would be a waste of time, the way you
are judging things, the sack of knowledge behind each and every thing you make is enough to know that you are facing a very fine
educated man and craftsman.
I simply adore your courage to face and combat the lies promoted by the huge "sharks" on the market, never seen this before and maybe I will never see it again; it requires arguments, self trust and motivation for the good of the customers. Once again thank you very much for all your efforts to share your vast knowledge with us! May God bless you for long and peaceful years in the Sharp Instinct Studio! :) All the best,
--A.


An important part of understanding steel phase transformations is the phase diagram. While scientists and metallurgists diagram many things, the phase diagram illustrates precisely under what circumstances individual phases form.
The key to understanding heat treating is understanding transformations, and our induction of these transformations. This is another reason I believe the knifemaker should do all his own heat treating, so he can precisely control the structures of his creations. This may not matter much if he's using plain carbon steels and hammering out his blades in an open air forge—rarely do bladesmiths who work this way use any method other than looking at a relative and generalized color of heated steel for their control. They look at the color of the heated steel and make a judgment. There have been attempts to substantiate this process by giving it a technical sounding name like "thermo-optical emission viewing" (whoa!), but it's dependent on the skill, references, background lighting, color sensitivity, and the material itself, and is simply a guess. This is not how the best blades made of modern high tech alloys are created; there are instruments called pyrometers that can measure temperatures to a fraction of a degree.
The knifemaker who works with the highest alloy tool and stainless steels should be part scientist, or at the least, a laboratory technician, able to produce specific, controlled, and regulated environments and exposures for accurate and repeatable results in his steels. While I do make some forged blades and use pattern-welded damascus steels from time to time, these are chosen for one reason only; the patterned appearance. The very best performers are, of course, high alloy stainless and specialized tool steels.
The diagrams that detail these specific phase and eutectoid transformations specify points at which the eutectoid transformation occurs, which are the points at which one solid transforms into two different solids with different properties and compositions, important to understanding the whole process and how critical temperature is.
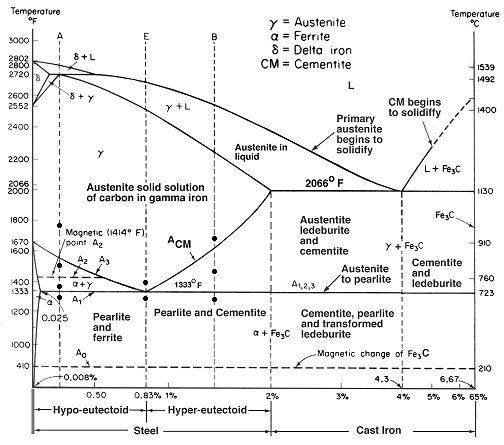
While at first glance, the chart seems intimidating, for fine modern high alloy tool steel blades, we are only concerned with the narrow band of hyper-eutectoid steels. The first thing to remember is that these are equilibrium charts, and that the phases show here occur in slow temperature changes. Start at the very top of the vertical dashed line labeled "B." B is in the middle of the hyper-eutectoid band of the steel area listed on the bottom of the chart.
With this basic understanding of the phase diagram, I encourage you to look over and examine different areas and indicators on this and other diagrams. They are a rather simplistic way of showing how materials transform from liquid to solids, the eutectic points of steel and iron, and the temperatures at which all of this occurs. There are charts showing sublimation, deposition, melting, freezing, condensation, vaporization, and the crystalline structure of all kinds of materials.
Note that martensite is not on this chart, anywhere. This is the really important point here. This chart is describing material transformations at equilibrium, which for steel and iron, means very slow temperature changes. By sudden and deep cooling, we alter this slow migration of carbon and iron, and form astounding structures that drastically affect the steels performance and arrangement.
Hi Jay,
Thanks for the great site, sharing your beautiful knives, and your knife knowledge and philosophy.
I was raised as a mechanic and welder in my family’s heavy equipment business and 30 years ago, the
knowledge you are freely sharing was handed down father to son and not shared to the world.
My wife is a professional pastry chef, food blogger, and teacher. I thought she needed a custom
knife for valentines or her birthday and looking at all the $500 ‘customs’ I thought, hell I could
do better than that I have a metal shop in the garage. I started reading the knife forums and the
usual drivel about real knives being forged. Being disabled, my hammer swinging days are over.
Then I ran into your site. I spent the last week and a half studying as much as of your site as I
could digest and as importantly as how, the why's.
Thanks again for the copious knife knowledge, I help my wife on her blog so I know how involved
building and maintaining a site is. If I was blessed with riches, instead of free time and enough
knowledge and tooling to be dangerous I would put her name on your list for gorgeous Concordia,
instead she will get a well-crafted RogboBilt O1 chef and paring knife, plain but made with love.
Best,
Roger

When water freezes to ice, it happens at exactly 32°F/0°C. At this same temperature, ice turns to water. It's so accurate that thermometers are calibrated in a bath of crushed ice and water because it's exactly 32°F/0°C. This is not true with steel (iron with more carbon), and when we add more carbon, the weirdness factor increases. Steel starts freezing (solidifying) at one temperature and is completely frozen at a lower temperature. So it has a range of solidifying. In this range, steel is mushy like oatmeal or mud. Start adding more carbon, and the range of mushiness gets larger, until about 2% carbon. Add more than 2% carbon and the range of mushiness gets narrower, and then the range goes away at just over 4% carbon.
Solidification is not the only physical action where steel changes. Steel, when heated below the melting temperature to certain specific and critical temperatures, undergoes changes in the internal crystalline lattice structure. These structures are called phases. The magnificence of steel is based on these phase changes or transformations.
The word "eutectic" comes up often in steel discussion, but the word itself simply means "easily melted" in Greek. It may help to understand various applications of the word in the study of the science of materials to clarify the idea of eutectic principles in steel.
Back when I was very young and working in industry, we did a lot of welding. I worked as a maintenance electrician, mechanic, and instrumentation technician. This sounds like a lot, but mainly consisted of a small group of guys handling every single device, machine, driver, power feed, control, and regulation problem in a small-to-medium sized manufacturing plant. This meant wiring up new devices, troubleshooting failed systems, welding broken components, tuning and calibrating every machine and device the plants needed. I worked in half a dozen different plants like this: a plant that made concrete coated steel pipe, a fiberglass manufacturing plant, a secondary aluminum smelter, a pigment manufacturing plant, a circuit board plant, a radio crystal manufacturing plant, and even a large electrical generation station. Though I took vocational welding classes when I was still in high school as an advanced student, the classes were only a brief overview and introduction to real world welding issues we would encounter in the plants.
One of them, a seemingly simple one, was how to weld stainless steel to mild steel, or stainless steel to a high carbon, high alloy steel. A machine would fail, or corrode, and stainless parts were needed because, wisely, the plant maintenance manager wouldn't want to have to shut down the production line and fix the problem (due to corrosion) again. We came up with special welding rod made by Eutectic Castolin®. This is a great company that's been around quite a while, and makes some neat stuff, essential to maintenance and repair as well as welding advancements.
Now, each metal in the bond (lets say high alloy steel and stainless steel) each have a high melting point, and their melting points are different. But when you mix the two metals together in just the right percentages, you get an alloy that has a lower melting point t than both the parent metals! It's an amazing thing, and the welding rod from Eutectic Castolin® already had this perfect mixture in the rod. So when you welded with this stuff, it bonded to both parent metals at a lower temperature than they would ordinarily melt at, allowing a great penetration and bonding of the weld flow.
Simply put, eutectic transformation is a liquid cooling into a solid that has two phases.
Steels aren't the only metals to have eutectic properties. When I got into jewelry work, I quickly learned that eutectic bonding of dissimilar metals was necessary in all parts of the work. For instance, copper has a melting point of 1984°F/1085°C. And silver has melting point of 1763°F/961°C. If you try to fuse copper to silver, the silver will melt and dribble away long before the copper melts. However, if you mix up an alloy of 28% copper to 72% silver, the alloy has a melting point of 1431°F/777°C! This is great! This means that with that alloy between the pieces of silver and copper, you can solidly bond the two dissimilar metals at about 300° F below the lowest (silver) metal's melting point!
Metal alloys aren't the only materials that do this, and it's not only about melting, but also about the phase transformation of a solid. Understanding that in eutectic concentrations in steel means that several components in a specific combination create a whole that has a lower critical temperature than the individual components.
In the combination of steel, the elements iron and carbon, depending on their percentages in relation to each other and the temperature, give some steels strict eutectic points. To get gritty about this, the two atomic species form a joint super-lattice based upon their valence electrons.
To throw another term into the mix, we have eutectoid steel. Eutectoid simply means "eutectic-like," but In this case, the word eutectoid describes a process of phase transformation where one solid forms into two different solids. Steel with 0.8% (actually 0.77%, but let's round the number) carbon can transform (with heat) to austenite, and in equilibrium cooling, austenite can then undergo complete phase transformation into pearlite (cementite and ferrite) without a transition zone and without any extra ferrite or extra cementite. This is considered eutectoid steel.
Hypereutectoid high alloy steels are complex steels requiring complex treatments.
Steels with less than 0.8% carbon are called hypoeutectoid steels, and hypoeutectoid simply means a mixture of components having less of the minor component (carbon) than the eutectoid composition. Hypoeutectoid steels can transform in the same equilibrium cooling phase transformation to pearlite and ferrite. Ferrite is a soft component abundant in mild steel. Railway track is a great example of hypoeutectoid steel. So are railway spikes. You might want to consider that when you see a knife made of a railroad spike. As knives, they are ornamental only, containing carbon in the range of 0.15% to 0.30%, creating soft, weak blades at their very hardest. But some folks like the look and they are easy enough to hand-forge.
Steels with more than 0.8% carbon have so much carbon that they transform into cementite before the eutectoid point and they are called hypereutectoid. Hypereutectoid means a mixture of components having more of the minor component (carbon) of a eutectoid composition. Hypereutectoid steels can transform in equilibrium phase transformation to pearlite and cementite, with many more abundant hard particles of cementite. Cementite is created because of the high carbon content.
These steels are therefore harder, more wear resistant and more durable in long-term use as knives and cutting tools. This is because the higher carbon can form abundant carbides. These carbides are not only iron carbide, but with high alloy hypereutectoid steels, are also molybdenum carbide, chromium carbide, and vanadium carbide, and combination carbides of multi-elements. Also, high carbon creates a more profuse and abundant martensite formation, which, after tempering, creates a stronger, tougher, more wear-resistant steel overall.
The important difference in these three types of steel in knife blades (hypoeutectoid, eutectoid, and hypereutectoid) mean more than just the carbon content, though that is the defining factor. The crystal morphology is different in all three of these steels. Hypoeutectoid steels form Windmanstätten patterns in their ferrite side plates projecting into the austenite grains. The microstructure of hypoeutectoid steels will then contain Windmanstätten ferrite and fine pearlite.
Hypereutectoid steels form a continuous network of hard, brittle cementite along the prior-austenite grain boundaries, and are typically only used where extreme hardness is required, such as in cutting tools.
In all types of steels, the ultimate tensile strength increases with increasing carbon content. However, yield strength varies little with increasing carbon content.
The only advantage of using a lower-carbon hypoeutectoid steel is increased ductility, which means a softer knife blade more likely to bend than break. This is not the reason to make a fine knife blade; cutting tools cut because of high hardness and wear resistance. Hard edges cut; ductile edges bend and dull.
Most chef's knives and kitchen knives made and sold today are made of hypoeutectoid stainless steels because they are inexpensive lower alloy, lower carbon steels. Being available in sheet stock, it's easy and fast to punch-press them into knife blades. They only require simple and fast heat treatment that is usually automated, and are easier to machine, grind, and finish. They also dull much faster, requiring constant steeling, honing, and sharpening with traditional methods.
A good use for a ductile blade is a machete, since it will likely encounter rocks and high impact. But making soft, ductile machete blades is really not the realm of the maker of fine knives, because soft, ductile steel blades are a dime a dozen at any hardware store, big box warehouse, or lawn and garden outlet.
Hypoeutectoid steels have also been extensively used in structural steel applications, and were produced in extremely large tonnages. However, even the hypoeutectoid steels are being replaced in structures by High Strength, Low Alloy (HSLA) steels because of higher toughness.
Hypoeutectoid steels are limited in function, and simply make inferior knife blades. They do forge well, and that is their main attraction in this field. As I've detailed before, hand-forged blades are mediocre in every performance aspect when compared to blades that are machined, offhand, in stock removal method using hypereutectoid steels, particularly with lengthy, detailed, and high performance cryogenic heat treatments.
If you're familiar with this website, you know that all of the steels I use in knife blades are hypereutectoid. They have a lot of carbon, for good reason. You won't see this terminology used by knifemakers and knife manufacturers because they are often using hypoeutectoid (lower carbon) steels, and thus revealing this doesn't sound so great. Even in the stainless steels, hypoeutectoid types are the more common players in handmade knives. Steels like AEB-L, 13C26, and 14C28N that are commonly used for knives are low performing hypoeutectoid steels, having only 0.67% carbon.
Hypereutectoid steels are harder to heat treat, with more complex and necessary deep cryogenics for maximum transformation into martensite, and more profound development of allotropes. They are not steels that can be fudged, estimated, or generalized in their treatment, they must be handled with high accuracy and control of the process.
Just because a knifemaker uses hypereutectoid steels doesn't mean he's treating them right. Most knifemakers absolutely do not treat them to their highest potential, missing the marks on temperature (deep cryogenics required) timing (low cooling slope of time vs. temperature), and multiple temper cycles that have strict control with deep cryogenic equilibrium stages in between. Hypereutectoid high alloy steels are complex steels requiring complex treatments to bring to their pinnacle of condition.
The reality is that higher carbon hypereutectoid steels are the better steels for knife blades. They are superior because they have high carbon content. Whether or not they are stainless steels, high alloy steels, or just plain carbon steels, the amount of carbon is critical to their performance and value.
The words hypo-eutectic and hypoeutectoid, and hyper-eutectic and hypereutectoid are different and have different meanings depending on the country and language, as the United Kingdom and European English version of the word is not the same as the American English version, so research papers and presentation vary somewhat.
Hello Mr. Fisher,
I just want to thank you a lot for writing your long detailed page on heat treating.
After about 4 days of scrolling internet forums and such, your post laid it out the best.
So relieved...!
Thank so much for your time... otherwise all the best!!
Sincerely,
Marc Stanton
Thank you again- like finding the holy grail of treating that cut through all the floating opinionated stuff.


"Martensite is the most important constituent produced by heat treatments designed to produce ideal mechanical properties."
--George Vander Voort
Martensite and the Control of Retained Austenite
Metallography, Failure Analysis, Archeometallurgy Consultant
Martensite is probably the most important crystalline structure knifemakers and toolmakers are concerned about, so I want to go into it a bit more. Martensite is formed from austenite (gamma-ferrite) detailed above. When heated to transformation temperature (the decalescence point described below), the ferrite (alpha-iron) and cementite (iron carbide) transforms to austenite (gamma-ferrite). The body-centered cubic lattice structure of ferrite transforms to a face-centered cubic lattice structure, containing up to 2% carbon in solution. The carbon has migrated from the orthorhombic cementite structure into solution, and also into the face-centered austenite. Since carbon atoms are 1/30th the size of iron, there are extra carbon atoms in the lattice structure floating around in the interstitial spaces (the spaces in between the iron atoms in the lattice).
In slow cooling, the carbon atoms will drift back into the alpha-ferrite arrangement (body-centered) alternating with layers of cementite and stabilize. In high carbon steels this slow cooling produces pearlite, layers of ferrite with cementite as the carbon diffuses into coalescing cementite layers (recalescence explained below). The cementite is in platelets.
This is an equilibrium phase, where physical changes happen at fixed temperatures with the material at thermodynamic equilibrium, without consideration for time. In simpler terms, equilibrium means staying at the same temperature for a long time, or extremely slow changes in temperature. These phase changes happen in equilibrium by diffusion, where atoms move due to thermodynamic and internal motion. The carbon moving within the face-centered and body-centered interstitial locations is a product of diffusion. These phases are represented on an equilibrium phase diagram, but martensite is not.
The reason martensite is not represented is because these particular phase diagrams are for materials at equilibrium. Martensite is not an equilibrium structure because it grows without diffusion, from it's parent austenite, inheriting its chemical composition. It does this by displacive transformation. It's also highly strained, a kinetic product brought about as a result of rapid thermodynamic temperature changes, again, not at equilibrium.
In fast cooling, the carbon simply has no time to move very far, so it's forced into the crystalline lattices making a new arrangement called martensite. Martensite is actually a distortion of the body-centered structure, making the lattice into a strained tetragon (rectangle). The actual movement of atoms within the crystalline lattice structure is no more than the distance between two atoms. The crystal structure has lots of dislocations, which can be envisioned by plenty of forced angles from many directions, strengthening the steel.
So the crystals are—in simple terms—interfering with others, strained, twinned, and bent. I know, you metallurgists are wincing, but I'd rather not go into the details of edge dislocations and screw dislocations formed by shear and wave front motion in evolving crystalline bodies. It's enough to know that many dislocations create an irregular lattice, which is much harder to break.
The microstructure of martensite has no simple analogy that is easy to understand in our physical world. The science of steel is rather complicated, so, without getting into morphological and crystallographic characteristics, the strength of martensite (over the same steel untransformed) is caused by: dislocations, fine twinning of the crystalline bodies, smaller grain size, and movement and segregation of the carbon atoms. Additional strength is caused by the formation of carbides. More on carbides later.
Martensite doesn't form all at once on cooling. It has a start temperature (Ms) and a finish temperature (Mf). In this cooling range, austenite transforms to martensite. The cooling has to occur quickly, otherwise, the crystalline structure will convert to bainite, or if cooled even slower, pearlite, the layered alpha-ferrite with cementite. Slower cooling also allows the austenite to stay austenite, or be retained as austenite. While there is always some austenite retained and some converted to martensite, the amount is very important. In the scientific sense, there is no absolute martensite finish temperature; this is determined at 95% transformation, since complete transformation is not known. So, Mf =95% martensite transformation. The important procedure is transforming as much austenite as possible into martensite.
But what about all that extra carbon? In martensitic transformation, it's forced into small, chunky particles rather than the layers and platelets of cementite as in pearlite. These tiny, extremely hard particles become carbides in further treatment steps, and are spread throughout the martensite, and increase the wear resistance tremendously.
Simply put, martensite is similar to the body-centered cube ferrite (bcc), but instead of a cube, it's body-centered tetragon (bct) with more carbon occupying the spaces between the iron atoms. The transformation reaction is very fast, at near the speed of sound. Since the bct structure is less densely packed than the fcc structure of austenite, there is a volumetric expansion, resulting in hardening of the steel.
Did you know that:
"Martensitic transformation occurs in other metals and materials, not only in steel.
It occurs in, for example, nonferrous alloys, pure metals, ceramics, minerals, inorganic compounds, solidified
gases and polymer."
-H. K. D. H. Bhadeshia
Martensite in Steels
Materials Science & Metallurgy, 2002
Hello Jay,
I am just delving into knife making as a hobby. Your website is a treasure trove of valuable information that
has been a great reference for me. Thank you for investing the time to share your expertise.
Regards,
Charlie Ward Wright IV

These are curious properties of steel allotropes, and can help in understanding how carbon migration, phase changes, coalescence, and the whole heat treating process works with steels. Decalescence and recalescence are physical properties based on temperature, and may be used to describe or indicate the critical temperatures where these phasic changes take place. However, they are not something manipulated or changed in any way by the knifemaker, simply something that occurs. Not understanding these reactions may lead to improper, ineffective, or less than optimum heat treating.
As the alloy is exposed to heat, the actual temperature of the steel continues to increase. At critical temperatures, phase change starts to take place. At these points, the steel temperature does not increase, even while additional thermal energy is being supplied by the furnace. Instead, the temperature of the steel decreases as the energy is used to transform one phase of the steel to another phase. Applying additional heat will not increase the temperature of the steel, only encourage further transformation. When transformation is complete, the additional heat supplied by the furnace will then cause the steel to increase in temperature in a more normal fashion. What's important here is that the energy is being used to change the crystalline lattice structure of the steel. This property of absorbing heat energy while decreasing in temperature during heating as phasic change is underway is called decalescence.
In decalescence, the important thing to remember is that plenty of ambient heat and ample time in the furnace is necessary for the phase change to take place, and temperatures must be closely held and applied. This is why the best heat treatment takes place in an electric furnace with accurate pyrometers, regularly checked and calibrated. Too low of a critical temperature, or too short of an exposure to that temperature, and complete phasic conversion will not take place. The blades need to be removed immediately after full phasic transformation, and not heated further. This is another reason that hardening is not accurate in a blacksmith/open forge environment. In that environment, judging the time and temperature for decalescence is only an estimation. This is why no industrial, machine shop, or heat treating facility uses open forges (or blacksmiths) for this critical step.
A critical point is that the decalescence point should not be held too short, nor too long, and the cross-sectional geometry of the blade being heat treated plays a large role in this. A thin blade must not be held too long above this temperature, a thicker blade must not be held too short. This varies from blade to blade, and the only way to control this is for the knifemaker to have an understanding and complete control of the process with accurate equipment and devices in use.
If the steel knife blade is heated for too long or for too high of a temperature after the decalescence point, the carbon will start to migrate to the surface of the steel and bond with any free oxygen, decarburizing the steel. This is a serious fault in heat treating, and it mainly comes from overheating the steel for too long or for too high of a temperature, or exposing the steel to an oxygen-rich environment during heat treating. In the machinist's guide and in other heat treating references, decarburization is listed as the number one failure in heat treating steel!
In my own studio, I've used protective environments for decades, including vacuum, inert gas (nitrogen and argon) purged furnaces, and stainless steel foil wraps that create an oxygen-depleted environment around the blades. In all of these types of decarb protection, certain other steps are necessary to ensure the proper and immediate transformations take place, such as adjusted timing, quench methods, post-purged environments, and manipulations of the environment furnace and quenching equipment. It's critical in all steels that will be used as tools to prevent decarburization during heat treating. Yet, in open forges by blacksmithing methods, decarburization is expected as if it's normal!
This is important, because If a blade is decarburized, two things happen. The first is that a black, hard carbon-rich crust will form on the blade, and will have to be removed. This "scale" is composed of a significant portion of the carbon that has migrated out of the steel to the surface and bonded with free oxides in the atmosphere. Grinding, sanding, and removal of this crusty surface of the steel means extra effort, changed geometry, and difficult finishing.
This indicates the second and most severe failure of process in decarburization. The carbon content of the steel is lowered, meaning less martensite, less carbides, and a softer, less wear-resistant, and weaker blade overall. It is a very bad thing; truly a failure of heat treating. You might read that blacksmiths and unprotected furnace heat treatment creates a scale that has to be ground away to "get to" the steel underneath that is not decarburized, but the depth of the decarburized steel can only be estimated, and is uncertain and tenuous. This is why no professional heat treating organization uses an open-air or unprotected method.
Think of this. There is one critical place on a knife blade where decreased carbon in the steel would do the most damage. That place is the cutting edge. Since it's the thinnest place on a knife blade, it has the greatest propensity to decarburize. There isn't much steel there, and the carbon can deplete from both sides, dramatically decreasing the carbon in the alloy... right at the cutting edge.
There is no way for decarburization to be seen in a finished knife blade, no way to correct it if it does happen. The result is a blade that performs less than expected, has less carbon available to create carbides, and has lower wear resistance overall. This is because loosing carbon converts the steel to a lower carbon alloy! More about decarburization below.
This is the temperature slightly above the decalescence temperature that the steel is brought to in order to ensure that complete transformation has occurred. There are all sorts of interpretations of this temperature, some saying 50°F, some saying 100°F above the decalescence point, but it is truly only confirmed by experience guided by the manufacturer's white papers. Even the white papers may give several different hardening temperatures with the different results produced. Here, the experience of the knifemaker is key, along with accurate electric furnaces and calibrated pyrometers.
Recalescence is similar, only an opposite in physical reaction of decalescence. As the steel is cooled at critical points, phase change gets underway and even though the ambient temperature is dropping, the steel increases in temperature at these critical points. This is because a phasic change is happening. This is the steel trying to reach entropy, and it has to give up some latent energy while undergoing physical change to do this. So it heats up while cooling during phasic change as this change of state releases energy.
With recalescence, the same concerns exist as in decalescence, but with some more dramatic applications. Effective cooling will absorb latent heat during recalescence and phasic change, and enough coldness (absorptive environment) will allow the extra latent heat to be pulled from the steel, effectively aiding in phasic change. This is important in annealing.
However, in knife blades, during normal heat treatment, we do not want recalescence to occur! Recalescence is an equilibrium reaction, in other words, it happens in slow transformation, with austenite transforming to pearlite and ferrite. Unless we are fully annealing a blade, we don't want this to happen; we want martensite instead. In order for this transformation to occur, we need fast and immediate cooling so recalescence can not happen. This means an even more robust cooling environment with the ability to pull the heat from the steel at the most rapid rate possible without resulting in stress fractures or warping.
Because of these critical temperature reactions, it should become clearer that the most effective means to control this environment is an electric furnace and accurate freezers with calibrated and accurate pyrometers. To try to do this visually at the blacksmith's forge is simply a guess, which may be only a fair estimation in hand-forged hypoeutectoid blades. This method should never, ever be used in the treatment of hypereutectoid high alloy and stainless tool steels.
Titled: Knife Heat Treating Article
"Nice article. A college course in itself.
I have learned more about knives from your web site than anywhere else.
Those TV shows like 'Forged in Fire' are somewhat amusing now."
--R. S.


You'll undoubtedly read or hear about retained austenite (RA) in knife steel and tool steel discussions. No matter how a piece of hypereutectoid steel is treated, there will always be some austenite retained in the structure when it finally reaches room temperature. Everybody wants to limit or decrease the retained austenite because it's softer than the martensite or carbides in the structure, and decreases the steel's hardness after heat treatment. Retained austenite reduces the mechanical properties of the steel significantly, negatively influencing yield strength, machinability, fatigue strength, and even corrosion resistance. It even affects the size of the steel!
On the other side of the argument, some austenite is necessary for resilience of the structure and during tempering, the austenite is transformed to ferrite and cementite, toughening the structure overall. However, that is tempered austenite, not untempered austenite, and there is a huge difference.
Martensite expands when it forms, and any remaining austenite is surrounded and pressurized in small islands, with force exerted by the martensite expansion. In order for the austenite to transform to martensite, it needs to expand, but it's trapped. When the steel is brought into service, pressures and elastic deformation can allow retained austenite to transform to martensite, but the steel has already been tempered, so these tiny islands of untempered martensite are brittle and can cause fractures. Since it is on a microscopic scale, this would present in a knife blade as high wear at the cutting edge, exactly what you don't want in a knife blade.
Overall, knifemakers and tool makers want to reduce the amount of retained austenite, and increase the amount of martensite, giving a more durable structure that can then be tempered to the preferred balance of hardness and toughness that the blade and knifemaker, and ultimately the knife user requires.
Just what is that balance? It depends on the steel type, the intended use of the knife, the habits and application of the knife user, and the skills and understanding of process by the knifemaker. This is another reason a maker should, at the very least, heat treat his own knives. Sending a knife off to a heat treater who slaps a "58HRC" on the blade is a cheap and fast solution, but not participation of, understanding of, and result-based function of the knifemaker. It is not custom, it is not controlled by the maker, it does not demonstrate to the knife client that the maker has a grasp on the complexity of the process. If he doesn't have a grasp on the basic metallurgy of the blade, what does that say about the geometry of the blade, the fittings and fixtures, the handle? What does that say about his understanding of the knife use and application, durability, and longevity? What does that say about his practice creating the sheath, stand, or display? These are all parts of knifemaking, and they start with the blade.
Consider that chef's knives can vary in temper depending on the style, use, and preference. A boning knife may be favored by the chef to be from 55HRC (springy and flexible) to 62HRC (extremely hard, rigid, and wear-resistant). I've had clients requesting one of each! Knives used in combat and counterterrorism may range from shock resistant and tough to ultra hard, bordering on brittle, and every point in-between. This can depend not only on the user preference, but the blade and knife design and construction overall, including the features of serrations, armor-piercing thickened sections, or razor-thin recurve areas. It is a very delicate balance and it's only learned by experience and feedback, coming from years of custom knifemaking. So it's easy to see how a heat treating contractor farming dozens (or hundreds) of blades through his line at once cannot offer much in the way of variety or specific treatment.
How does one determine the percentage of retained austenite? X-ray diffraction, that's how (by ASTM and SAE standards). Since knifemakers are not typically in possession of this equipment, they must be educated on the standard practices and procedures to produce the desired levels of these structures in their shops and profession. The information is out there, available to everyone, and there is no reason anyone who makes knives and has a access to the internet should not have a grasp of this. Granted, most knife users are not interested in the amount of retained austenite in their knife blade, but they do want to trust their knifemaker or knife manufacturer to supply them with the best knife possible in their budget.
Simply put, we want to limit retained austenite to as small a percentage as reasonable, with as much of the austenite transforming into martensite as possible. It's pretty simple to perceive, but extremely interesting to understand how it all happens.
Your Web Site-
Dear Sir,
I am impressed; you are the epitome of a professional.
--Alan


The word quench comes from Middle English quenchen, from Anglo-Saxon cwencan, causative to acwincan: to decrease or disappear. The definition currently is to destroy, extinguish, or in our field, to cool suddenly, as in heated steel, by immersion in water, oil, or air. Rather than look at quenching as an act of extinguishment or decrease of the heat, we must take a different view; one of transformation in the suddenness of time. In my view, it's not the heating that is the amazing thing, it's the quenching, and the results of the nature, timing, medium, rate, and depth of the quench.
Quenching, like most aspects of this profession, is a balance. It's a balance of cooling the blade steel as rapidly as possible, but not so rapid that the shock of cooling causes cracking and fracture. Want to know what I mean? Take a piece of 440C high chromium stainless steel and heat it to 1900 F. Pull it out of the furnace and quench it in cold water. It screams, it cries, it shudders, it vibrates as if the hounds of hell are tearing at its soul. And then it shatters into dozens of pieces that settle to the bottom of the bucket. Okay, just a visual for you to consider, but fun, anyway.
The balance a knifemaker walks is one of sudden and deep enough cooling to transform austenite into martensite, but not so sudden as to so severely stress the blade that it cracks. The exact quenching method is specified by the steel manufacturer or foundry, so it's not some mystical question that has to be resolved. Many steels (like O1 oil hardening tool steel) are designated by their quenching medium (as are A2 for air hardening and W2 for water hardening). The designation is not consistent and sometimes a particular steel type can be quenched in several different mediums. For example, ATS-34 may be quenched by air or heated oil, with slightly different results. This is another reason for knifemakers to heat treat their own blades, as entirely different properties are derived depending on these steps.
You may note that the medium designation is followed by the word hardening. This is what quenching does, it hardens the steel. It does this mainly by suddenly transforming austenite to martensite, taking a blade from a soft, plastic, glowing hot mass of steel to a cold, hard, stiff, and brittle one.
There are three considerations in quenching, and together, they play a pivotal role in the character and structure of the steel knife blade. They are:
While the white paper specifies the recommended treatment and expected results, there are some issues with these documents and their presentation. More on that below. Generally, though, they are a reliable guide to processing individual steels.
For centuries the Swiss would take advantage of the extremely low temperatures of the Alps to improve the behavior of their steels. They would allow the steel to remain in the frigid regions of the Alps for long periods of time to improve its quality. Essentially, this was a crude aging process accelerated by the very low temperatures. What we now understand to have happened was the reduction of the retained austenite and the increase in martensite. By performing this once secret process the Swiss obtained the reputation for producing a superior grade of steel.
Lakhwinder Pal Singh, Jagtar Singh
"Effects of Cryogenic Treatment on High-speed Steel Tools"
"Cold" treatment was well known, both by process and results since the industrial revolution. Old Swiss watchmakers would "age" parts in the snow, and Pierce-Arrow would age engine blocks by putting them outside in the winter. There are other stories of men and companies aging and treating steels to the cold for advantage of higher performance on the market, but they simply did not have access to the modern investigational tooling and devices we have to identify the results of the process. They did realize verifiable known and positive results.
When I started knifemaking, there were no cryogenically quenched knife blades. Guys were just starting on the right track, and we used dry ice and freezers, acetone and alcohol, and whatever we felt improved the rate and depth of cold to harden the steel. Granted, the steels performed extremely well even without this extra step. To this day, if steel knife blades are processed according to the manufacturers recommendations without cryogenic treatments in conventional heat treatment methods, they will perform extremely well, because simply, the alloys are superior to plain, medium, and high carbon steels.
So why bother with cryogenic treatments at all? After all, it costs money, time, space, materials, electricity, and expendables to do cold treat. We do this because it produces a markedly better steel overall, and we can prove it, particularly in the high alloy and stainless steels.
When I started to expand, refine, and improve my quenching and heat treating process, I looked at it much like I look at all of the various facets of my work: improvement in steps. As I've explained in bio, at one point my blades were good, but my handles awful, so I tried to improve my handles. Then my sheaths were the worst, so I improved them. When I thought all of that came together, my embellishment was weak, so I worked to bring that up. Then, back to the blades, as the handles were now better than the blades. I look at this profession as one of continual improvement, constant upgrading and refining, coherently creating evolved works, and it was only natural to improve my steel processing as this went on. I started with no cold treatments, moved to long-term sub-zero freezing, then on to cryogenic treatments.
Let's define and identify these quenching treatments.
I'm sure there will be some discussion and emails about this, but the term cryogenic simply means the study of materials at very low temperatures. Some define the temperature delineation of cryogenics at -238°F, but it is an interpretation and not specific. Cryo means cold; a cryogen is a material or solution used for freezing. The word cryo comes from Greek kryo, simply meaning icy cold.
I'm using standard convention detailed by some modern industries, who are well-versed in the technology: The Journal of Materials Processing Technology, The American Iron and Steel Institute, the International Journal of Emerging Technology and Advanced Engineering, and other various research sources (some detailed below).
This, then, is the target range of temperatures of quenching for our field:
| Description | Temperature °F | Temperature °C | Achieved With |
| Conventional Heat Treatment (CHT) | 70°F | 20°C | Room Temperature |
| Sub-Zero Treatment (SZT) | -15° to -100°F | -26° to -73°C | Mechanical Freezer, Dry Ice |
| Shallow Cryogenic Treatment (SCRYO or SCT) | -121°F | -85°C | Mechanical Freezer |
| Deep Cryogenic Treatment (DCRYO or DCT) | -300°F | -135°C | Liquid Nitrogen |
Dear Jay,
Your knife site is frankly dangerous. I have lost myself for countless hours reading and ogling your
website and learned more about knives and knife making in the process than I thought possible. I especially
love your simple, clean and extremely verbose technical style.
In the world of knife making your site should be listed as a cultural treasure.
Regardless, thank you from the bottom of my heart for one of the best websites on the internet.
Sincerely,
Aaron Young


There are three critical factors in cryogenic quenching of steel:
Understanding these helps to grasp the whole process and what is happening to the steel.
1. Quenching Rate: How does one determine the rate of quenching? It's the fastest cooling possible without experiencing destructive results in the steel. These highly negative results are cracking, distortion, warping, twisting, and uneven cooling that produces cracking, distortion, warping, or twisting. To be specific, first we refer to the manufacturer's white paper and other online resources. Second, we use trial and error. Why is trial and error needed? Because most manufacturers supply information based on 1" thick sections of the steel. Most ASTM, AISI, and other institutional testing and evaluations are based on large, heavy, thick sections of the steels, and knives are none of these. So knives differ from rated and recommended processing because they are relatively thin. Knives in quenching must be handled quickly, with speedy movements and well laid-out and well-designed equipment helps a lot in this.
I remember having a relative in the studio heat treating his own knife. He was moving slowly, methodically, carefully, gently grasping the wrapped blade from the furnace, leisurely carrying it over to the table, cutting off the foil wrap, digging inside to extract the blade, all while I'm telling him, "Hurry, hurry!" He did not rush, and the steel quenched too slowly, and was barely hardened. We had to heat treat again. Speed is essential, and fluid, continual movement between the mediums is critical. Quenching has to be planned, thought out, even scheduled, particularly if multi-stage quenching to cryogenic temperatures is part of the process.
Specifically, in cryogenic quenching, the sudden and drastic exposure of the steel to shallow or deep cryogenic temperatures can impose such stresses and shock to knife blades that they can crack. Even if the crack is not visible to the human eye, too fast of a drop in temperature can have a detrimental effect on the actual crystalline structure of the steel with microscopic fractures, and that failure will present itself as high wear of the steel, and a markedly less-than-optimum condition. In order to quench at an effective and continuous rate, quench staging can be employed. Depending on the steel, an initial quench based on the medium (oil, air, water), followed by freezing to below zero, and then slow cooling to shallow cryogenic temperatures, and finally deep cryogenic temperatures, if required. The rate must be controlled carefully, and each type of quenching has specific means, specially designed devices, and equipment to control this rate so the cooling is continual, even, steady, and uniform for the specific cycle and range.
What is the specific rate of cooling below room temperature for most of these steels? 4-5 degrees Fahrenheit per minute. That means in order to reach -100°F, it should take about 40 minutes (from room temperature), and to reach -325°F should take about an hour and a half (from room temperature). This is why simply dipping blades into cryogenic baths of dry ice and alcohol or liquid nitrogen is a huge and destructive error, yet knifemakers who are uneducated in this process frequently do this, and tell others that it's the proper way to quench! Sad, truly sad for the knife client. The cryogenic process cooling rate is critical.
Like too fast of a quenching rate, too slow of a rate is not as effective. Remember that quenching quickly is the goal, and a continuous curve in the downward temperature scale is essential. This is because allotropes converting are metastable, and it's important to continue the cooling process without delay. While not as damaging or limiting as quickly dropping the blade into the deeply cold environment, the blade does need to keep cooling at a good rate. Being that knife blades are thin, relatively small pieces of metal, the cooling rate should be as fast as possible while adhering to that 4-5 degrees per minute Fahrenheit rate. Another consideration is uniformity in the cooling jacket, environment, buffer, and cycle. Once the rate is established and reliable, other factors can be tweaked, adjusted, and changed for a variety of distinct performance levels in the custom shop.
2. The Quenching Temperature: Again, a critical factor. This one is verified by pyrometer, and it's great to live in a time when we know, or can know, precisely what temperatures our blades are quenched to and when. In multi-stage quenching, each device or environment has a known temperature, and the instruments that measure this should be regularly verified and calibrated. This is another factor that doesn't typically happen with "thermo-optical emission viewing" (looking at colors in hot steel), typical in hand-forging works. The actual temperatures reached may be recommended on the white papers, and most steels have extremely narrow ranges of required temperatures (as quenched to) necessary for the expected results. Some of these steels quench in stages, some may have to be interrupted or even held at intermediate temperatures during the quench cycle.
3. The Quenching Hold Time (Cryogenic Aging): This is an extremely important factor, as steel transformation does not happen all at once. Curiously, one textbook written in 2006 claims that the hold time at quenching temperatures does not matter, simply that the temperature is reached. This is totally in error. It has been proven time and again that cryogenic aging is critical, and some studies (Lal) have found that the length of cryogenic soaking is more important than the temperature of cryogenic medium! The quenching hold temp/time is critical to success of the process, and special means and devices must be employed to sustain this. Most early experiments had wide and inconsistent results, and it was traced to this critical "hold time soaking," which varied so much amongst the scientists and metallurgists that they weren't even sure cryogenics was worth the trouble!
Key studies have shown that it is not enough to cool the steel, but that it must hold a good long while at these low temperatures to realize the benefit of cryogenic treatment. Carbide development, nucleation, and precipitation is a sluggish reaction, and steels continue transformation for a substantial period after reaching the lowest temperature. Experimentation has shown the limits of this and what is also too long to be reasonably beneficial. What are the cryogenic aging times? 6 to 36 hours or more, depending on the steel chemistry, size, geometry, and expected results. This is another reason to keep the process in-house, to assure times are met and not shorted for the sake of economy.
People have asked me about what appears to be a conflict of process about the hold time at cryogenic processing. Here's a part of an email from a man trying to navigate his way through the processing:
In your link I just read a recommended soak time of 6 to 36 hrs. Would you suggest the full 36 hrs of soak time? I find it ironic that I often read how important it is to perform the first temper immediately after quenching to reduce chances of experiencing stress cracks, yet some of the latest heat treating studies for knives suggest deep freezing (which imparts more stress on the steel) for as long as an extra 36 hrs of high stress.
I can see how this can be confusing, and I want to be clear. When I claim that the soak time is 6 to 36 hours, it's a generalized statement. I'll break this down for clarity:
First, this is a minimum recommended soak time, and actual soak times may be much longer. In my own work, if it's possible, I've maintained some blades at 60 hours or more. The time is the minimum, and what happens is that the improvements taper off, and eventually, it becomes more costly to hold the knives in this environment than realize additional benefits which would offset the continued cryogenic soak. Refrigeration, liquid nitrogen, the cost of continuing operation of the processor, and the practice of creating the super-cold environment outweighs what beneficial results happen. After a few days at this temperature, not enough gains are realized to justify the expense. So, as long a soak as possible, but don't put the steel away for weeks, there isn't enough improvement after a few days to justify the cost of the process. Percentage points of improvement are noticeable, but when fractions of a percent take days to achieve, it's not worth the cost.
The second part of this statement is the wide range (6-36 hrs.) I've included this range because of the variety of steels used for knife blades that may require cryogenic processing. Not all alloys are the same, and each one requires a different regime. I keep a detailed log of all my process operations and results, and that's the best way to hone in on specific, repeatable processing.
The third bit of confusion is understandable. In one section, you read that tempering cannot wait; it needs to happen immediately, and then you read that the blades are held in the cryogenic processor for days before tempering! The statement that tempering cannot wait is first based on studies of conventional heat treatment. Quench a steel like D2 to room temperature, and you'll see a hardness of 62HRC, but leave it at room temperature overnight and it will be 52HRC! So, in conventional heat treatment, you can see how tempering must not wait. The longer the time the steel is kept at a temperature between room temperature and 100°C (68°F to 212°F) after the complete transformation of martensite, the more likely the occurrence of quench cracking from the volumetric expansion caused by isothermal transformation of retained austenite into martensite.
What about in cryogenic processing? It's important to understand that room temperature is not cryogenic temperature. Leaving a blade at a static 68°F is not the same as holding it in an environment of -325°F. At room temperature, allotropes are metastable; they will change in time with (or without) mechanical pressure; this metastable nature is why it's critical to get tempering underway immediately. At cryogenic temperatures, steel is not metastable to any degree that would affect a change in the structure, apart from compression which forces precipitation of carbides at nucleation sites. In other words, conversion is still taking place. Once the steel blades are warmed to room temperature; all bets are off, and tempering must take place immediately, or the allotrope will convert. Again, the longer the time the steel is kept at a temperature between room temperature and 100°C (68°F to 212°F) after the complete transformation of martensite, the more likely the occurrence of quench cracking from the volumetric expansion caused by isothermal transformation of retained austenite into martensite. I hope that clearly explains the differences between holding at room temperature and compressing and converting at cryogenic temperatures.
The warm-up rate for the blades from deep cryogenic temperatures (-325°F) to room temperature before tempering is important to know. This is done by just letting the blades sit in still, room temperature air. On this page and several others of this website, you'll see photos and a video of my blades on racks with condensate (steamy-looking cold air) falling off of them or blades on a rack covered with dense ice. This is how they warm up. It's not a good idea to slowly warm them (leaving them in the cryogenic environment to slowly reach room temperature as the processor or environment warms); this may allow metastable austenite to convert. Conversely, hurrying the process along by sticking them in a pre-warmed oven is also not a good idea, since this is highly stressful. The balance between these is simple; they are pulled from the cryogenic environment, sit in still room temperature air until condensate on them turns liquid (above 32°F), and then moved into a cold oven to evenly ramp up to first tempering temperature.
To know just how each factor contributes to the steel improvement, I'll cite the studies from a scientific test of a low carbon martensitic stainless steel used in piston rings.
So what does this study show us? First, considering the steel used by the scientists, this is a fairly low carbon martensitic stainless steel, and I wish they would have done their test with a higher carbon alloy. What we do know is that the effects of this treatment are even more intense and amplified in the higher alloy steels, and this is one of the lowest possible improvement rates. Even so, it shows that the cryogenic temperature was the most critical factor, followed by the aging (soak) time, then the cooling rate, and finally variations in tempering temperature played the lowest role. Remember, the result we are looking at is increased wear resistance, and this is why temperature, time, rate, and overall processing is important to knife blades, as wear resistance is the characteristic we are enhancing. What are the improvements in higher alloy steels? They can be up to eight times the wear resistance of conventionally treated steels!
It's extremely important to know that the processing of the steel during heat treat is one of the largest successful or detrimental factors in blade steel performance. Along with geometry, the knife blade's performance is a result of the knifemaker's understanding and employment of steel heat treating process. While people in this field often generalize the relative performance of steels based on anecdotal tales, poor testing, and popular gossip, most inferior blade steel performance is based on the geometry of the blade and the processing during heat treat. Many, many steels perform well, and properly processed high alloy steels are the very best steels we have access to in the modern world.
Hi,
I just wanted to take a moment to thank you for sharing your vast knowledge with the world at large,
and providing people like me so much insight into knife-making and metallurgy for free. Even for someone
like me who may never be able to afford one of your masterworks, the knowledge you've shared has already
made me a wiser consumer where knives (and possibly tools in general) are concerned...Thanks again for all the knowledge and inspiration. If I ever do strike it rich, or end up spending most of my
time outdoors, I'll be sure to add even further to your list of back-orders.
Much Respect,
D. G.



To the right is a photo of the interior of the cryogenic freezer. This freezer pre-chills and offers an environment to reduce the latent heat energy (or "scavenge the cold") in pre-chilling buffered knife blades. It also maintains the very cold environment for shallow cryogenic freezing and equilibrium reactions.
How cold is this freezer? -125°F or -86°C. The interior of this chamber is relatively large, and it's interesting to note that this mechanically refrigerated environment is significantly colder than every type of dry ice slurry used by most knifemakers. On the section below on dry ice quenching, I detail the many important and limiting factors of dry ice quenching of knife blades in great detail.
125 degrees below zero is very, very cold. It's so cold that I don't dare touch anything, surface, walls, inside panels, door, etc. when I access the freezer. If I do so without protection, my skin would immediately freeze to the surface, with horrifying results. The temperature of this freezer is very close to the very coldest recorded temperature on earth, recorded at the Soviet Vostok Station in Antarctica on 21 July, 1983. It was 128°F below zero.
It's humorous to think that people want to spend time on Mars, where -125°F is the average temperature. Sure, it might reach up to 70°F at the equator during the Martian daytime, but that evening, you'd better get ready for a bit of chill as in the same location, the night will be 100°F BELOW ZERO. And, since the atmosphere is 100 times thinner than earth and is mostly carbon dioxide, there's not a lot of potential for basking in the holiday retreat. Still, people think we need to go there. They should try establishing a thriving colony on Antarctica first, to work the bugs out of the process.
Closer to home, my freezer is big enough for one man to fit inside, yet no one seems to want to experience this nightmare—I could even evacuate the atmosphere for a more realistic effect.
The objects you see in the freezer are the Dewar bottle for liquid nitrogen storage, and the apparatus that contains the actual knife blades. Holding these items in the freezer makes the liquid nitrogen and deep cryogenic environment last a very, very long time. The interiors of these are -320°F or -196°C: incredibly, terrifyingly cold. Yet, since the "exterior" of the apparatus is -125°F, the cold environment lasts a long time. This allows me to perform extended, continual, and intermittent process steps at this deep cryogenic equilibrium state.
The frost you see in the freezer is the result of air entering when the door is opened. The moisture in that air chills and condenses snow, squeezing the water out of the atmosphere. This photo was taken in the dry times of our New Mexico winters, when the humidity in the air hovers between 10 and 15 percent. Much more frost occurs in the summer months, when the evaporative cooler is running. Snow in summer; I like it!
"Cryogenic treatment improves tool life of cutting tools, decreases cutting forces and wear of tools, enhances surface roughness, fatigue resistance, and ductility. Studies show that cryo-treatment improves abrasive resistance, thermal and electrical conductivity, tool life, hardness, toughness, and wear resistance."
--The Effects of Cryogenic Treatment on Cutting Tools, Satish Kumar, IOP Conference Series: Materials Science and Engineering, 2017
The sections above have outlined the particular factors of cryogenic quenching in a general way. No specific results were described, and if you wish to dive into the technical side of what happens and what we know (and some of what we don't) this section will help clarify why knife blade steels that are properly cryogenically treated are better performers overall.
Cryogenic treatment results offer more than just a larger volume of martensite in the steel; the cryogenic treatment even increases toughness, which is counter-intuitive to most conventional ideas of what happens during this long, cold cycle. Modern study and the capability to examine the micro-structures of steel with improved microscopy and related testing equipment have given us new and continual insight into this amazing process. The process is so fascinating, an in such an evolving state that new research is needed and is currently underway. This means that modernly processed knives are not only the best they have ever been in history because of alloy content and manufacturing methods, but also the best because of treatments available. With equipment crossovers and secondary market of equipment sales, these processes are available even for small volume knifemakers. What an exciting time to be making knives!
First, let's look at high carbon steels (hypereutectoid steels) for the basis and reason for sub-zero (SZT), shallow cryogenic (SCT), and deep cryogenic (DCT) treatments. It's important to know that the most significant improvements in steels are achieved in shallow cryogenic treatments, and somewhat less dramatic initial improvement in deep cryogenic treatments. Further enhancements happen in long, extended cryogenic equilibrium soaking, and even more improvements happen with cryogenic sequences between temper cycles. Since the exact results vary depending on the steel type, equipment arrangement, and protocols for each phase of treatment, this is another reason for the knifemaker to heat treat his own blades.
With these factors, even medium carbon steels benefit immensely from cryogenic treatment, and the effects are more profound in the high alloy, high carbon steels.
Thank you for everything you have taught me via your website. I try to follow your philosophy and guidance on every one that I make. I'm no Jay Fisher, but I'm getting better everyday. Thank you. No need to reply. I know you are busy.
--Kevin Zito

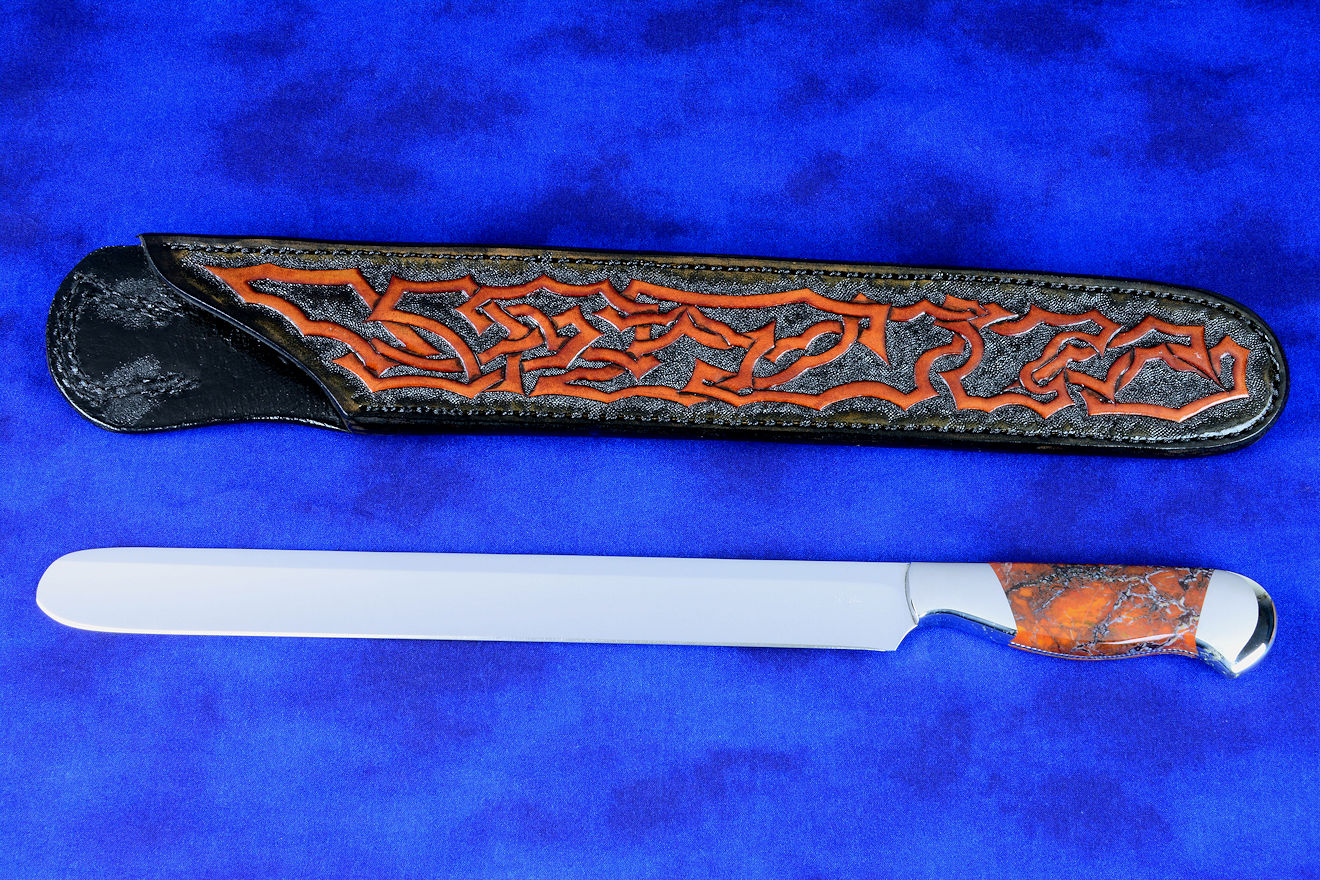
For complete transparency, please note that since my first knife made in the 1970s, until the present day, I've heat treated every single one to the best of my knowledge and ability. Know, also, that I've never had one failure, not one return, not one complaint about the hardness and wear resistance of a single knife blade I've made.
Heat treating is not mystical wisdom, not a mystery of scientific knowledge, and not an unobtainable goal: it is simply a process. It's hot, it's cold, it's timing, it's workflow. It's numbers, it's temperatures, it's logical, like any process. And like any process, understanding, control, record, and repeatability are the keys for reliable results.
In order to better understand what happens in the entire cryogenic heat treating process, and to illustrate more specifics of the results, it's important to detail tempering. Tempering is part of the heat treating process that is performed after quenching and cryogenic aging. There is a lot of myth about tempering, and abundant misconception, confusion, and even error where the words "cryogenic" and "tempering" are used in the same phrase, for there is no such thing as cryogenic tempering.
It's noteworthy that some companies sell cryogenic processing equipment called names like "cryotemper" or "cryofurnace." These descriptors are their names for cryogenic processors married with tempering ovens that cool the metals to cryogenic temperatures and hold them at those temperatures for cryogenic quenching and aging, followed by draining of the coolant and slowly heating to tempering temperatures for automated tempering cycles. Understand that these are separate functions, all done in the same machine, thus the blending of the words "cryo" and "tempering." Still, there is no such singular operation as "cryogenic tempering."
Tempering is the process of re-heating the steel to force transformation of a percentage of the crystalline structure into another structure. The reason to temper is fairly straightforward. If steels (knives in particular) are left in an as quenched condition, they are far too brittle and unstable for working tools. All properly heat treated tool steels must be tempered to increase toughness and plasticity, and reduce brittleness and stresses. It isn't because the hardness is too high in untempered steels; it's the lack of toughness and the lack of thermal conditioning resulting in further crystalline microstructural changes in untempered or improperly tempered steels, and these changes can cause micro fractures and cracking, even on a microscopic scale. This presents as lower wear resistance, or a less than optimum cutting edge retention. Additionally, important carbides are formed in the tempering cycle, carbides critical to improved wear resistance.
Tempering is necessary to reduce stresses, balance the hardness and toughness of the steel, and increase wear resistance by the development of complex carbides. It works in several ways:
Bad tempering practice will negate any well-executed hardening process.
While the description above was fairly short and sweet, you might need to know what happens specifically in tempering, and why tempering metallurgy is so important in knives. It's the knifemaker's responsibility to control these critical steps in the process, because the design, geometry, and ultimate use and longevity of the knife exists in the tempering control. Miss the temper and no matter how well the blade steel was hardened, no matter how costly and time-consuming the cryogenic process, the knife blade will not be at its optimum condition. So many knives are shorted in this process, with missed steps, incomplete conversion, or inappropriate cycling steps, time, and temperature, that the cryogenic process of hardening is not recognized. Simply put, bad tempering practice will negate any well-executed hardening process. In this section, I'll describe exactly what happens during temper and you'll understand why this is so important.
Stored energy of martensite—this is an important concept. The structure of martensite is carbon-rich. Remember that martensite is a body-centered tetragon, and thus has much less carbon locked into the crystalline structure than austenite which is face-centered. The sudden changes of quenching force carbon into the steel solution, which represents substantial energy. Carbon can migrate in steels even at ambient temperature because it moves so easily.
Once the steel has been fully hardened, with proper treatment, we can be assured that we have as little as possible of retained austenite. We have an extremely hard, brittle, penetration-resistant piece of martensite-rich steel, with abundant and profuse complex eta-carbides and alloy carbides.
How is temper set and what exactly happens during this process?
Temper happens in ranges and stages. These ranges are not specific points, but broad transition ranges that overlap considerably. The temperatures listed below are generalized for higher carbon steels, but it’s critical to remember that increasing alloy content will raise these ranges and retard the tempering process. The alloys that do this are chromium, silicon, manganese, molybdenum, and vanadium.
Range One: The first range is from 200-500°F (95-260°C), and specific ranges vary with steel alloy type. When the steel blade is heated to this range, more unusual carbon movement in the alloy occurs. The carbon atoms move toward lower-energy or “hungry” sites in the crystalline lattice, like dislocations. Some of this movement has already happened during quenching, particularly in lower alloy, lower carbon steels. In fact , in low alloy steels, 90% of the carbon has redistributed to these low-energy areas while martensite transformation was occurring. In high alloy, higher carbon steels, more substantial carbon migration takes place during tempering. Carbon moves to these low-energy dislocation areas and begins to coalesce during tempering. These atoms then group together to form transition iron carbides, mainly epsilon-carbide, which has a hexagonal structure and eta-carbide, which has an orthorhombic structure.
You might wonder why the carbon atoms wouldn’t simply bond with ferrite and form iron carbide like our cementite structure. Actually, this can happen, with the carbon directly converting to cementite, if the defect density is large. This is why it’s so important to eliminate defects with good steel hardening process, and using clean, high quality steels. You would be right, then, to question hand-forged and forge-welded (pattern-welded) damascus steels, due to the obvious high defect density created in making them.
Because the excess interstitial carbon has moved out of the martensite, the surface energy decreases; the martensite is more stable and less subject to fracture. In the higher alloys containing chromium, vanadium, niobium, and molybdenum, a small increase in hardening (supplemental hardening) can and should occur. This happens because the structure can be initially refined in tempering by filling of the dislocations with carbon clusters. At these cluster sites, alloy carbide precipitates of these heavier, larger atoms (Cr, Mo, Nb, V) grow. This is different from secondary hardening which happens in some alloys at a higher temperature range. At this stage, it's more of a refinement of the microstructure and initial building of the carbides.
Simply put, in the first tempering range, these things happen:
Range Two: The second temperature range is 400-600°F (205-315°C) and specific ranges vary with steel alloy type. In this range, some retained austenite can convert to ferrite and cementite, and this adds ductility to the steel. If the proper martensite finish temperature was reached, there will be little retained austenite, but there is always some, and it’s important to convert it to the more stable ferrite and cementite. Ferrite created in tempering is a tough, ductile component and the cementite is iron carbide and hard, but the important thing is that both are stable. For our particular use, in some high alloy hypereutectoid steels, some retained austenite can convert to martensite on cooling from tempering. In effect, this is like quenching, which demonstrates why it’s important to quench out of the first temper stage into cryogenic range, to make certain that complete martensitic conversion takes place as the martensite finish temperature is again reached. This also means a second temper stage must occur to temper the newly-formed martensite. Often, a third temper stage is required for complete conversion.
Simply put, in the second tempering range, these things happen:
Secondary hardening range: In high alloy hypereutectoid steels, often a secondary hardness effect occurs. This happens mainly in steels containing molybdenum, chromium, and tungsten. Secondary hardening is a precipitation effect as cementite-rich carbides are replaced by fine alloy carbides. The temperature range at which this occurs depends on the alloy that is forming the carbides, but it's a relatively high temperature, often around 1000°F (538°C). This is a critical, beneficial range for alloys that must work at elevated temperatures and retain extremely high hardness, often around 70HRC. Some of the alloys are designed just for this critical range and application of extremely high hardness at elevated temperatures. In knife blades, this is detrimental. Steels treated for secondary range hardening suffer a tremendous loss of corrosion resistance which is usually why high chromium alloys are used in knife blades to begin with. They are also more brittle in this range.
Range Three: The third range begins at 500°F (260°C) and specific ranges vary with steel alloy type. Understand that in extremely high alloys, this temperature range is often above 1200°F (650°C). In this range, high complexity of movement and transformation occurs, and not all of it is good, particularly for steels to be used as tools! A transitional and metastable carbide, chi-carbide is formed. It has a composition that is between epsilon carbide and iron carbide (cementite). Increasing the temperature more, and the transition carbides start to convert and disappear, and are replaced by simple iron carbide (cementite). When this happens, martensite starts to lose its shape and changes from a tetragon to a body-centered cube. At dislocations, the cementite coalesces and nucleates, then starts to form spheroid shapes, which reduces the surface energy considerably. The ductility in the steel increases. The spheroidite grows, and smaller particles dissolve. The higher the temperature at this point, the softer the steel becomes. Eventually, as temperature increases, the martensite disappears as it’s converted to ferrite.
Simply put, in range three tempering, these things happen:
The kinetics of tempering can be very confusing. Most knifemakers and heat treating companies simply heat the steel up for a while, and let it air cool, thus partially transforming some of the steel, and the result is adequate enough. This is not understanding or refinement of the process, and this is where the individual knifemaker who has knowledge of the process can excel.
As each cycle of tempering is completed, the ranges determine the stages of transformation.
I hope that sentence makes sense, but if not, please read it again. The reason I am determined to get these concepts across is so that you who are reading this will understand that each cycle of tempering brings a certain and detectable refinement in the microstructure. After quenching, there is a certain condition, and after the first tempering cycle, there is a different and improved condition. The same thing happens after the second temper cycle, and these improvements can be detected. After the third cycle, an even further enhancement of the condition can be seen if the process is very good.
Few people look for or can notice these improvements, but they do occur when the entire process is refined, and the knife owner and user will definitely notice them in the condition of the knife blade itself as it is brought to its premium condition!
Alternate method: Or, you can just heat the blades up in a campfire of pennantia Baylisiana (the King's Kaikomako). This has to be done slowly under cover of darkness, because it's the rarest tree in the world, with only one living specimen. Then, certain chants must be performed from the Necronomicon Ex-Mortis (hats-off to Bruce Campbell). Quenching the blades in Westvleteren 12 ale, acquired from the gates of the brewery, will give you the perfect knife blade condition that we are all chasing.
And you thought tempering was so simple...
Performing a "snap temper" is tempering immediately after conventional quenching. The blades are quenched to room temperature, and then put in a tempering oven at an elevated temperature for the purpose of converting some of the newly-formed martensite to ferrite and cementite and bainite, thus softening the steel!
Why do this? It's done because in some cases, the stress of continued quenching to cryogenic temperatures can crack the metal. But this is only typical on large and irregularly-shaped metal pieces such as forming dies, which may have very thick and very thin areas. Knife blades are not forming dies, and it's been my experience that they cool uniformly enough that snap temper is not necessary, and is detrimental to the steel allotropes formed.
Several people have asked me if I ever had any cracking of blades in stress riser areas (around holes, filework, in radical geometrical changes of cross-sectional areas) in any of my blades. This is a great question, because heat treating contractors will tell you that this is the reason to perform a snap temper. I'll be very clear on this: I've never, ever, had one single crack of any kind in cryogenically processing any knife blade steel! Not a single incident. Take a look at some of the radically shaped blades I make, even with full engraving, and know that not a single incident of stress fracture has occurred. This is because metallurgical references state that snap temper is done for these reasons:
From this, it's clear that since knives are not wildly complex dies with thick and thin areas, the reason to snap temper is number two, three, and four: convenience and liability. Know that this is detrimental to the final steel allotropes and condition.
I believe that heat treaters who do this do it because it's easier and safer to take this step so that the likelihood of fracture is lessened, since the blades do not belong to them, and they don't really know the alloy makeup or properties (as they are simply told this by the person who sends them the blade to heat treat). Also, consider that heat treatment contractors do not typically know who the manufacturer or foundry of the steel is, and there are some variations of treatment between steel blades coming from different sources.
One more consideration. It may be possible that the heat treating contractor is not using proper processing protocol. He may be simply dipping the steel right into the super-cold environment (usually liquid nitrogen) without following the 4-5°F per minute rule. If he snap-tempers first, it's unlikely that he will get a fracture since the steel is no longer in complete quenching, because it's partially converted to ferrite. This is not a good practice and demonstrates a failure in processing strategy.
Additionally, the longer the time the steel is kept at a temperature between room temperature and 100°C (68°F to 212°F) after the complete transformation of martensite, the more likely the occurrence of quench cracking from the volumetric expansion caused by isothermal transformation of retained austenite into martensite. In treating large batches of steel, the snap temper allows the heat treater to take his time in the process, by removing stresses that could be caused by volumetric expansion. He can fit the cryo portion of the process into a more convenient time schedule. This may be particularly necessary if different types of steel are heat treated, since the furnace times and temperatures are different, yet the cryogenic treatments are the same. To do large batch processing, it's simply more economical and safe... for the heat treat contractor. This is detailed in the topic about batch processing and cost factors below.
For these reasons, I believe that performing a snap temper is a safer, cheaper way for the outside heat treating contractor to reduce the possibility of fracturing someone else's knife blade, and thus, incurring financial loss. It also may be a way to hurry the process, costing the contractor less to treat the blades. We can rely upon scientific testing that shows that snap temper, when necessary, permits less conversion to martensite, a lower density of martensite, and a lower density of carbides, curtailing performance, simply for the sake of economy. This is no way to treat a premium knife blade steel.
This makes me wonder, then, if this is another source of why so many are skeptical of the process overall. Just like the inadequacy of a proper cryogenic aging time, results can be less than optimum with a snap temper, which is usually only necessary for irregular shaped items with both thick and thin areas, like metal forming or plastic injection molding dies, or when the timing of immediate cryogenic processing is inconvenient for the person processing the knife blade.
"Cryo-treatments are, clearly, the most effective in improving wear resistance if applied right after quenching rather than after tempering."
Zbigniew Zurecki
Cryogenic Quenching of Steel Revisited
Air Products and Chemicals, Inc., 2005
Tempering several times or multiple tempering is a critical process application, and should be carefully researched and performed according to manufacturer's white papers and based on the design criteria of the knife's actual expected use and geometry. Why is it important to have multiple tempering operations?
The properties of these high alloy steels are dependent on their individual microstructure and lattice components, which are created and refined during the entire heat treatment cycle. The final hardness alone is not the determinant factor of that microstructure, the entire process is, and from this section on tempering, you can see the role that accurate, meticulous processing plays in that structure and the ultimate durability, longevity, and value of the knife blade.
Hello Mr. Fisher,
Thank you for your willingness to share your knowledge through your website. I have learned so much and have had my view on knives permanently
altered by the knowledge I gained from reading your website.
I will begin the same way as many of letters you receive by saying “Thank You!” Your website and the information you provide are extremely appreciated. Factory made knives are ruined for me now that you have provided a framework for me to logically think through what they are offering. I will admit that I was taken in by what you refer to as the “mysticism” of the knife industry until I read your site. I am a mechanical engineer for an aerospace company and as I read your site, all of your arguments were logical and matched to everything I had been taught in school. My whole perspective on what a good knife is has changed.
--T. S.


The entire reason for having most of the refractory alloys in tool steels is carbide development. If a knife or cutting, machining, pressing, shearing tool is made of plain carbon steel (standard steel) or low alloy steel, no matter how high the carbon content, the focus of the steel's makeup and the heat treating is directed toward martensite development first.
Let's look at standard steels (plain carbon steels) to establish a starting point for this idea. In these steels, martensite is the desirable allotrope, and in order for the highest amount of martensite to be developed from austenite, all of the standard steels with carbon content over 0.3% must be quenched below zero degrees Fahrenheit. This means that standard steels with the designation 10XX, starting with the steel 1035 and up, must be quenched to well below freezing to reach the martensite finish temperature. No matter the quenching medium—oil or water—these steels must continue quenching to below freezing for complete conversion. Understand that most knifemakers don't know this, and don't do this, so they are settling for reduced performance and retained austenite, an undesirable allotrope.
In the standard steels, 1084 is the eutectoid steel, with 0.8% carbon, and I wouldn't even consider making any knife blade with a standard steel with less carbon content. This is because hypereutectoid standard steels (1086, 1090, and 1095) have excess carbon that encourages the development of iron carbides. Beyond the development of martensite is the creation of iron carbide from this excess carbon. In hypereutectoid standard steels, martensite plus iron carbides are developed, and in hypoeutectoid standard steels, martensite and ferrite are developed. Clearly, for knives, hypereutectoid standard steels offer vast performance improvements over hypoeutectoid steels.
If martensite and iron carbide offers good hardness and wear resistance in tool steels, why bother with the complicated and expensive process of cryogenics to create carbides of alloys? Why are high alloy tool steels created at all?
Alloys in steels are put there to improve many properties. What are they?
Carbide development improves all of these properties. Carbides developed in dislocation areas and grain boundaries improve elastic strength (preventing deflection) and shock resistance by inhibiting crack propagation. Mainly, they improve wear resistance and edge retention. They do this because they are hard, much harder than iron carbides, much harder than martensite. Here's a simple chart that details this clearly:
| Carbide Type | Rockwell C (HRC) Hardness |
| Iron Carbide | 64-68 |
| Chromium Carbide | 75-80 |
| Molybdenum Carbide | 75-80 |
| Vanadium Carbide | 85-90 |
| Titanium Carbide | 90-100 |
| Tungsten Carbide | 75-80 |
| Niobium Carbide | 90-100 |
From this, you can see that carbide development is critically important in tool steels, as all of the carbides are much harder than iron carbide. The Rockwell scale is not linear, so these hardnesses are substantial. Consider that there are multiple types of carbides with one elemental alloy, and these all differ in hardness. Also, some carbides are developed in multiple elements, such as chromium-vanadium carbides or chromium-molybdenum carbides that have tremendous hardnesses.
You might wonder why some of the alloys are used in tool steels and not others, and some are limited. This is because they have other properties or limitations. Niobium, for instance, is very limited in solubility in steels, and tungsten offers high heat resistance. Molybdenum offers increased toughness and chromium vastly improves corrosion resistance. Titanium looks promising, but inhibits the growth of chromium carbides. There are numerous properties to consider in any high alloy steel.
The important thing is that all of these alloy carbides are much harder and more durable than simply iron carbide, and much harder than the martensite if a knife blade is tempered to 58HRC, 60HRC, or even 62HRC. Carbide development surpasses martensitic structure when considering all of the properties desirable in a good tool, and a good knife blade.
Why wouldn't a knifemaker offer these alloy steels to his clients? This, then becomes the question.
What are the drawbacks, the limitations, the restrictions in using these alloys and directing heat treating toward high carbide development?
Jay,
I wanted to thank you for your for your amazing website! I have spent countless hours reading and
rereading your pages and get lost in all the information you provide. I have learned so much from
your pages and knowledge in the knife making world. So I wanted to say thank you from a new knife
maker to THE master of knife making! Your knives are impeccable and your knowledge and techniques
unmatched! I strive to, one day, be able to make a knife half as good as the great Jay Fisher!
Thank you for everything you do for the knife making world!
Chad Filler


Cryogenic processing dramatically and significantly increases toughness of all steels that are hardened and tempered.
There are a lot of misconceptions in this field, and knifemakers themselves spread most of the myths about toughness. How is it that a misrepresented proposition applies to knife blades and toughness? After all, aren't steel properties well-known and proven by testing?
Here's how this goes: in hardening and tempering steel, a balance between hardness and toughness must be reached and applied for each type of steel, each particular geometry and design of knife blade, and the intended use (application) of the knife by the knife user and owner. Generally speaking, if steels are treated to be harder and more wear-resistant, they lose toughness. If steels are treated to be tougher and more fracture-resistant, they lose hardness (and thus wear resistance). You can get a basic idea of this physical trait of balance on my "Blades" page at the bookmark, "General Hardness Table."
The knifemakers and knife supply companies (mostly on forums) perpetuate a straw man argument. They do so because they believe or insist that there is a zero-sum game in knife properties. They claim that if a steel is made to be hard and wear resistant, it must be less tough. Conversely, if steels are made to be more tough, they must sacrifice wear resistance. Ergo, all hard steels are brittle, all softer steels are tougher than the harder steels. This is a gross generalization that is perpetuated to sell a particular steel, usually a lower alloy or heat treatment protocol overall.
Please think carefully about who is making this claim and why. On one forum, a person claimed to have contacted a metallurgist from a high alloy steel foundry. The metallurgist assured him that toughness was increased in cryogenic processing, and the guy selling the straw man argument insisted the metallurgist didn't know what he was talking about. The problem was not the steel; it was the metallurgist who actually worked for the foundry! Wow.
This happens a lot in knifemaking, and it's hard for technically-minded and educated people to take a knifemaker seriously who makes such unsubstantiated claims. In plainer English, this is one reason much of the public thinks of most knifemakers as stupid hicks.
Just because a steel is hard and wear-resistant, it does not lack toughness. High alloys, properly treated, excel in every property: hardness and wear-resistance, toughness and resistance to fracture, lower asperity and higher corrosion resistance, increased stiffness and reduced elasticity, and increased longevity and useful application overall.
Thanks for helping to stop misconceptions, wives' tales, and falsehoods in our tradecraft, science, and art through education. For more information, please read some of the references listed below.
Dear Mr. Fisher,
I hope my e-mail finds you in good health. I have recently read on your webpage that you are recovering
from a surgical operation; I wish you a fast and successful recovery.
I am writing to you to thank you for your work, and for the works of art that you are creating, and for all the great knowledge on your webpage and all the inspiration you have given me. There are many beautiful, decorous knives with great fit and finish, but many of them are impractical and only decorous. Your works are beautiful, "clean" (not overfilled with details) and intended to be used. In my opinion they are just the best.
I am a young knifemaker and your works give me inspiration to make possibly the best knives. I will need many many years to achieve the quality anywhere near to that which you are making, but I will try my best. The problem with making better quality knives is that I don’t have anyone to talk to about them, to learn from or to find solutions of some the problems. Most of the other knifemakers in my country stopped their development at bushcrafts and somehow excluded me from their "community." The few that are making anything better just don’t want to share any knowledge. I wish I could visit you one day to talk about knives and learn something from you (I really have many questions), but I know that it is rather impossible. Neither have you have the time nor I money to travel to USA. I will be glad if you find time to at least read my e-mail.
Today, after several hours of hand-finishing very hard hypereutectoid stainless steel and trying to achieve mirror finish, it came to my mind that you would understand the pain of this laborious process and that and I should write to you and thank you. Without you I probably wouldn’t be putting so much work into every knife and wouldn’t be making them as well as I am right now (compared to what I did several years ago).
You don’t know me, but you are my mentor and teacher and the most important thing that I learned from you is to constantly develop and strive for the best. I wish I could learn directly from you, but I am glad that I can at least read your words, watch your videos and see photos of your works.
Thank you Mr. Fisher! I wish you and your family all the best!
With best regards, B. G.

These are modifications of conventional heat treating involving interrupted quenching techniques, or more than one quenching medium. This is done to minimize distortion, prevent cracking, and decrease the potential for other stress and conversion problems. I will reveal that these processes have no place in hypereutectoid high alloy and stainless steel knife blades, but may have a place in lower alloy and lower carbon steel types.
Austempering is heating the steel to its critical transformation temperature, and then quenching in a hot medium, usually molten salts, that are high enough in temperature (above the Ms point) to form bainite instead of martensite. This is not typically desirous in knife blades, since martensite is the desirable allotrope. It is typically done industrially to increase shock resistance, not something necessary on most knife blades as wear resistance is diminished from conventional quenching and tempering.
Martempering starts the same, with quenching in a high temperature medium (usually molten salts), and then removing from the medium to allow the steel to cool in air, so that martensite can form.
Both of these processes are then followed by actual conventional tempering after quenching has completed, which brings me to a curious point. Why are these processes called aus-and mar- tempering? The are, in actuality, processes that happen in quenching, not actual tempering of the steel. So maybe they should be called ausquenching and marquenching, as this is more accurate to the step in which these modifications are performed! But this is the terminology, such as it is, and this may be another one of the reasons there is so much confusion in the metals trade about these terms!
In any case, both of these process modifications to quenching have a result. The elephant in the living room is that both of these processes result in high levels of retained austenite, most undesirable in knives! Retained austenite reduces wear resistance, reduces strength, and leads to deformation as the steel is placed in service due to the problem of mechanical transformation, dimensional variations, and distortion at room temperature. All of these results are unwanted, and totally unnecessary for the knife blade heat treating process.
Austempering and martempering do have their place, in ductile and white cast irons, in high silicon shock-resistant steels, low alloy carbon steels, and some specialty metals, but I don't see any advantage of either of these processes in the treatment of a durable, wear resistant knife blade. The only reason I can see performing this (based on extensive testing and scientific results published by researchers) is to make an inferior steel (1095, 52100) more shock resistant than conventional heat treating process.
When you read the advantage details of austempered and martempered steels from companies who sell this service, you'll see why it's done. Most of these use entirely automated processers, and that is why they are economically preferred. From the austempering and martempering industry, the advantages are:
The steel types typically austempered and martempered are SAE 1045 to 1095, 4130, 4140, 5060, 5160, 52100, and 6150, distinctly low alloy steel types. Since these steels are inferior in many ways to high chromium, high alloy martensitic stainless steels, I don't use typically use them. Now, when you see the process identified, you'll know more about it and its applications in the world of hand knives.
Hi Jay,
I'm writing merely to thank you for the vast amount of information you've put up on your website.
I am an engineer professionally, and make knives in my free time. I learned everything I know about
knifemaking from the internet, and a significant portion of that knowledge came from studiously reading
your site (many times over.) The technical knowledge you've published was extremely helpful, and your
work itself is a huge inspiration.
I also took a page from your book and decided to "give back" - I wrote an extensive report on a
telescope I built, in the hope that it would help others in the same way that your website helped me.
Thank you again!
--R. S.


These are, technically, softening processes for steels, but their application depends immensely upon the alloy type!
Normalizing has no place or purpose in processing high alloy and stainless tool steel blades.
Read enough about knives and knife blades on the internet, and you'll come across the term "normalizing" sooner or later. Just what is normalizing and how does it work? More important, does it play any role at all in working and processing modern, high alloy and stainless knife blade steels?
Hey, just what is normal? With steel, everything is variable and changeable, so there really is no normal, so let's just get to the definition of the process of normalizing.
Normalizing is heating the steel to a temperature above the transformation range (where alpha-ferrite and pearlite convert into austenite) and then cooling it in still air. That's it.
What? Why? I will firmly declare that in working with high alloy hypereutectoid stainless and tool steels, we call this "hardening!" Yep, if you try this with any of these steels, they won't become normal, they will become quenched, and extremely hard.
So what is the purpose of normalizing? The purpose is to soften the steel and reduce stresses to make it more workable! What? I'll soundly declare that if you try this with these upper-tier high alloy, hypereutectoid and stainless steels, you won't be doing any work with them at all, as they will be in the lower 60s in Rockwell hardness, and a file and drill bit will just glance off the hardened surface. So in working with high alloy steels, normalizing has no place or purpose at all.
Normalizing is then for lower alloy steels and carbon steels.
Normalizing is a process that's cheaper and a bit faster than annealing, but based on the same idea. You take the steel to its austenitizing temperature, and then cool it slowly. But "cool slowly" is a general term, and needs to be suited to the individual steel alloy. For instance, in one reference, normalizing is done at 100°F temperature drop per hour, in another just sitting in room temperature air. The purpose of normalizing is the same as annealing, to reduce stresses or hardened areas before machining or working the steel. But the normalized blade is not annealed, and the properties of the steel are not uniform (as in annealing). Because normalized steel is not uniform, stress are created, and then the blade may then need stress relieved. So normalizing is not a final condition, but a part of the working process of a typically lower alloy steel.
Normalizing is done with lower alloy carbon steels as a cheaper and faster alternative to annealing, since it doesn't take as long and is not as expensive as having a dedicated oven slowly lowering the temperature of the blade in many, many hours. Because normalizing is not really effective in extremely high alloy tool steels, and annealing is, the word normalizing is an indicator that the knife blade is a lower alloy type. In all my decades of making fine, high alloy tool and stainless steel knife blades, I've never had to normalize a single one of them. Oh, I've annealed a few, but I can count them on one hand. The important thing to note is that high alloy and stainless tool steels are not normalized, they are annealed, and diligent efforts should be made so that annealing is never needed.
Annealing is full softening of the steel. In annealing, the steel is taken to its austenitizing temperature or a recommended temperature just below it, and then cooled very slowly, extremely slowly, to allow the equilibrium transformations to take place. Every process temperature, time, and step of annealing is different depending on the steel alloy content, and the white papers (technical data sheets supplied by the steel supplier) are a guide to this fully softening process. Annealing is done to create the most ductile, most malleable steel possible, for several reasons.
The important thing for me, as a professional. is to never have to anneal a blade in the first place! Both of these scenarios happen because of other failed steps or mistakes (bad process control or dull cutting tools) and I never, ever purposely want to have to anneal a knife blade. I'll also clarify that in some of these steels, full annealing is almost impossible; they stubbornly refuse to return to the state they arrive from the foundry (fully spheroidized and annealed). This is another reason so many knifemakers do not like working with high alloy tool steels and stainless steels; they are unforgiving of error or casual attention. They need to be made right, the first time, and processed once, for correcting an error may not even be possible.
Spheroidized metals are in their fullest, dead soft condition, and this is typically how they arrive from the foundry. The term spheroid refers to the spheroidization of the plates of cementite contained in the pearlite structure, making them big and round and granular and thus, ductile and easy to machine. Spheroidizing is a step beyond annealing, and is expensive to achieve, as it takes many hours in the furnace with extremely slow cooling so the equilibrium phase transition can take place. In spheroidizing process, the steel may need to be held for an extended time at the austenitizing temperature, and cycled in the higher ranges before extremely slow cooling results in a fully spheroidized structure.
Usually, spheroidization is not necessary, in the decades I've been making blades, I've never had to attempt this on a single one. Since most steel billets arrive at the studio in this condition, they are already at their easiest working condition, dead soft, and as soft as they are ever going to be. This might be surprising, though, to those who work with low carbon or low alloy steels, as even in their dead soft condition, these high alloy and stainless tool steels are comparatively tough and difficult to machine.
Now that you know these three important conditions of metal: spheroidized, annealed, and normalized, you'll understand why steels are shipped from the foundry or supplier in "fully annealed and spheroidized" condition, the reasons for annealing, and why no high alloy steels and stainless steels are ever normalized.

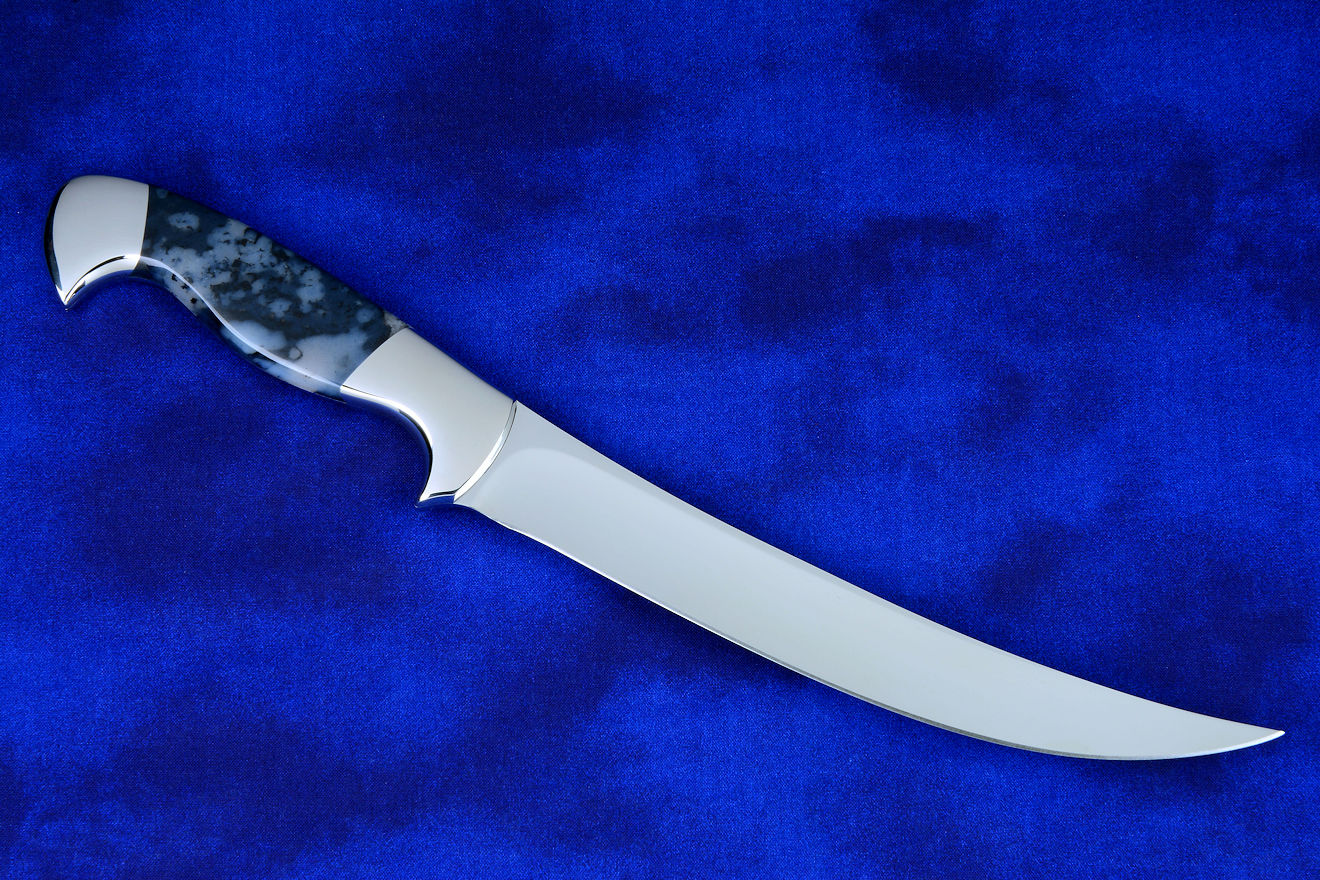
"When compared with classical quenching, performed to ambient temperature, cryogenic cooling has more effect on the steels with larger amounts of carbon or alloying elements."
Handbook of Residual Stress and Deformation of Steel, ASM International, Totten, Howes, Inoue, 2002
Stainless high alloy steels are the fastest growing steel types made. This illustrates how important these steels are to the world. Martensitic stainless steels constitute the majority of high alloy hypereutectoid steels I and other makers of fine handmade knives use, simply because they are the very best. Even without cryogenic treatment, their performance, strength, wear resistance, corrosion resistance, and durability overall surpass all lower alloy commonly hand-forged steels by many times and in all characteristics. High alloy modern tool steels, martensitic stainless steels, and powder metal technology tool steels benefit greatly from cryogenic processing.
While this information is still being studied, and not all of the effects are well-understood, it's clear that the performance of these steels is terrifically enhanced by cryogenic treatment. It's best to break these properties and results down into individual aspects that have been proven by studies and scientific experimentation:
O1: This high alloy oil-hardening tool steel is a standard in the industry for a reason. It's a great hyper-eutectoid tool steel with about 0.9% carbon and the version I use has high tungsten and vanadium with a bit of chromium, though not enough to be stainless steel. I use it when clients want a great performing black colored blade, because the finish and bluing is excellent on this steel. On one website about steels used in woodworker's tools, the writer claims that because O1 has a higher martensitic conversion than other steels, cryogenic treatment is not effective. This is flat-out wrong. While O1 does perform well with conventional heat treating, cryogenic treatment vastly improves this performance. How does it benefit from cryogenic treatment? O-1 can have up to 8.5% retained austenite when quenched to room temperature (20°C). While this does not seem to be a lot, it is significant, and proves that at the very least, O1 should be quenched to sub-zero temps and held there to reduce the amount of retained austenite. So much for the woodworker's assessment of O1. Now here's the really important result and proven by highly specific and controlled technical scientific studies: in treating O1 to shallow cryogenic treatment (SCryo), the wear resistance was improved 221%. In treating O1 to deep cryogenic treatments (DCryo), the wear resistance was improved 418%. Simply put, either of these treatments dramatically and substantially improves the wear resistance while making the blade tougher, and the finish better overall! Why not do this?
440C: This high alloy martensitic stainless steel is a great performer. In SCT, its hardness is increased by 4%, and in DCT, by 7%. and by bringing this steel to a shallow cryogenic treatment, it has 128% improvement of wear resistance. There is some conflicting information and data about 440C in the cryo field, with claims that deep cryogenic treatment does not significantly improve this steel performance. These claims would lead you to believe that 440C doesn't need the -320°F liquid nitrogen quenching of DCRYO. One study claims in deep cryo, it only has a 121% improvement of wear resistance, so it's assumed that 440C has better results in wear resistance improvement in shallow cryo. However, there's a very important thing that is seldom mentioned by researchers who evaluate these things, and something I've learned from working with 440C in both shallow and deep cryogenic processing. When 440C is processed in deep cryogenic processing, with multiple tempering and DCRYO immersion between tempers, it's markedly more dimensionally stable in DCRYO than in SCRYO. It's also tougher, stiffer, and much more wear resistant. I know that much of this research is done without extended long-term cycles and soaks at deep cryogenic temperatures, and one of the reasons it's so important to document and continue testing. In the future, look for longer-term cyclic testing on this steel; the performance using my T3 process is over-the-top outstanding, and I'll be documenting this as time permits. It's interesting to note that the standard by the United States Air Force for all parts made of 440C in any aircraft are that they are cryogenically processed, and that has been the standard since 1995!
D2: This steel is an incredible steel and benefits undeniably and astoundingly from cryogenic processing. Since D2 has so much carbon 1.7% and so much chromium (12%), it creates abundant chromium carbides even with conventional heat treating. The martensite is also profuse in the structure, due to the extremely high carbon. In shallow cryogenic treatment, D2's wear resistance is increased 316%, which is wonderful. In deep cryogenic treatment, D2 becomes another animal. DCT increases the wear resistance of D2 up to 820%! Over eight times the wear resistance of conventional or even sub-zero heat treatment is an astounding result, and it has been proven over and over again in numerous scientific studies how profoundly D2 responds to this procedure. If you have a conventionally treated D2 blade, you already know that this is a very wear resistant alloy, one that takes and holds an edge for an incredibly long time. Now imagine the same species with eight times more! When properly done, D2 also benefits from the precipitation of finer carbides which lead to increased toughness as well. D2 benefits from multiple tempering cycles (at least three), because they promote the precipitation of secondary carbides, and with triple tempering an increase in toughness of 25% is experienced when compared with double tempering.
ATS-34 (and 154CM) is a great performer all around. When given conventional heat treatment, it results in a high performance blade with high toughness and very good corrosion resistance (though not as good as 440C). When cryogenically treated, some very interesting results take place. The steel as in all high alloy martensitic stainless steels, develops extremely fine microscopic carbide particles. The finish is smooth and excellent, and because of the high molybdenum, two additional results occur. The first is that the creation of molybdenum carbides is abundant, particularly when given a good, long cryogenic aging. This brings up the hardness significantly and the wear resistance is abundant. The second benefit is that because of the high toughness in this alloy due to the high molybdenum, the blade can be tempered to a higher hardness overall, without fear of brittleness. The result is an extreme improvement of high wear resistance and high toughness, along with improved corrosion resistance. This same result can be experienced with 154CM, since technically, they are the same alloy. Since steels like D2 experience up to an 800 percent improvement of wear resistance with cryogenic treatment, In other studies of high molybdenum, high chromium, high alloy tool steels it can be suggested that a 200 to 300 percent improvement of wear resistance may be experienced, even though no certified testing data is available for these steels in the current research literature. My own research has demonstrated tremendous improvements in these steels when cryogenically treated, so why not do this?
CPMS30V, CPMS90V, CPMS35VN, N360: In all of the other steels I use in making knife blades, the cryogenic treatment certified testing data has not been done. This is probably due to several reasons; mainly the proportional rarity of these steels to common machine tooling steels, and the expense of the studies, along with requests from the steel suppliers for study details. Considering the typical results using high speed, cold work, and high alloy martensitic stainless steels, it is generally expected to achieve a minimum 200 percent increase in overall wear resistance, with up to a 800 percent increase in wear resistance possible with deep cryogenic treatment of these steels. Why not offer this treatment for clients who want the very best condition possible of their blades?
It should now be clear why cryogenic processing of these particular steels is one of the most important improvements that can be implemented on a high performance, high alloy steel knife blade.
En primer lugar me disculpo por el idioma en que escribo pero quisiera, si es posible, que la idea a expresar contenga en lo posible lo que siento y pienso.
He leído con atención una aclaratoria que hace en su página sobre sus experiencias, estudios y no menos valiosos conceptos de lo que su trayectoria ha demostrado en lo que hace y sus retractores.
No es mi costumbre mesianizar a las personas pero considero un acto de infinita justicia el reconocimiento no solo a la excelencia sino a la humildad y los deseos por contribuir a la enseñanza que usted ofrece y promueve.
A lo largo de la historia se ha demostrado que siempre es mas fácil descalificar y destruir(siempre que se pueda) que aprender mediante el esfuerzo y la constancia y eso, lejos de lograrlo con usted produce el efecto contrario y pone en evidencia al falso y el mediocre. Honestamente no pienso en la maldad de éstas personas sino en la incapacidad y la consecuente mediocridad.
Desde hace mas de diez años he seguido con atención su trabajo y sus progresos, creo haber mencionado alguna vez como admiro la unión familiar que usted tiene y el notable ambiente que se respira en su estudio-taller. Aunque es todo un espectáculo tecnológico y hermoso sus creaciones de cuchillos y hojas en general, hay otras actividades conexas que también merecen un reconocimiento y admiración como es el cuero, la piedra, la fundición, la fotografía la creación, diseño y construcción de maquinas y herramientas y lo impecable y útil de su sitio web...son muchas mas lógicamente.
Ya le he agradecido antes pero no me parece redundante repetir mi reconocimiento a lo que con mucho sacrificio y dedicación ha logrado en todas éstas décadas.
Reciba mis saludos extensivos desde luego a su familia..
Lic. Juan Herbut G.
Translated:
First of all I apologize for the language in which I write but I would like, if possible, that the idea I express should contain as much as possible of what I feel and think.
I have read attentively a clarification that makes on your page about your experiences, studies and no less valuable concepts of what your career has shown in what you do and your retractors. It is not my custom to messianize people but I consider an act of infinite justice the recognition not only of excellence but also of humility and desire to contribute to the teaching that you offer and promote.
Throughout history it has been shown that it is always easier to disqualify and destroy (whenever possible) than to learn through effort and perseverance and that, far from achieving it with you, produces the opposite effect and exposes the false and the mediocre. Honestly, I do not think about the badness of these people but about the incapacity and the consequent mediocrity.
For more than ten years I have followed your work and your progress with attention; I think I have mentioned sometime as I admire the family union that you have and the remarkable atmosphere that is breathed in your study-workshop. Although your creations of knives and blades in general are a technological and beautiful spectacle, there are other related activities that also deserve recognition and admiration such as leather, stone, casting, photography, creation, design and construction of machines and tools and the impeccability and usefulness of your website ... and many more logically.
I have thanked you before but I do not find it redundant to repeat my recognition of what you have achieved with great sacrifice and dedication in all these decades.
Receive my extensive greetings of course to your family ...
Lic. Juan Herbut G.


Here's a query about a particular steel (D2) and the cryogenic holding time:
Hi Jay.
I’m sorry to bug you so much regarding heat treating. But you seem to be extremely knowledgeable on the
subject of heat treating, and I appreciate having intelligent dialogue with you on the matter.
I looked up TTT curves for D2 last night. I read that for D2 the Mf temperature is in the range of -112F to -166F.
I remember that you mentioned that at -321F chemical transitions are very sluggish or slow…hence requiring extended time in this very cold state.
“the reaction of carbide precipitation is extremely sluggish, taking many, many hours. I know of no knifemaker who is holding the
blade in the dry ice bath for 10, 20, or 30 hours, and this is what is necessary for the most beneficial carbide precipitation”.
So, if the reaction/precipitation time is directly proportional to temperature, (in theory), would it be better to only bring the blades down
to ..say 170F and hold it at that temperature and consume less Nitrogen? At this temperature, all the Martensite should be converted to Austenite,
and the chemical reaction might be faster…requiring less soak time. I guess the problem is that I can’t quantify how much faster the reactions would
be, and I might be missing out on the Eta Carbides that form in colder temperatures.
Thanks again,
G. N., P. Eng
Senior Maintenance Engineer
This is a great query, because it's an opportunity to clarify some effects of the process, to the best of my understanding and experience, from what I have learned in study and practice. The first thing to note is that the Mf of D2, the temperature at which martensite is finished forming upon quenching, is about -150°F. Each steel is different, so it's best to make sure that one knows the makeup and recommendations of the individual foundry or steel supplier first. Even to finish martensite transformation, the temperature of -150°F must be reached, and this cannot be done with dry ice or even with shallow cryogenics. D2 is definitely a steel that needs deep cryogenic processing at -325°F for proper heat treating. Think about this when you hear or read that D2 is worked in an open forge and quenched and tempered in an archaic forge setting! D2 that is hand-forged is ruined steel.
I believe that Mr. N. suspects that conversion takes place slowly, sluggishly because of the temperature, and this is not quite the concept. The beneficial reaction of carbide precipitation is caused by the temperature, so I'll go into it a little bit deeper here.
Martensite formation is what happens when the steel quickly reaches its martensite finish temperature. This may not be a true cryogenic temperature; in the case of the D2 described above (-150°F), it's colder than the shallow cryo (-125°F) but not as cold as deep cryo (-325°F). At the martensite finish point, the steel has as much martensite as it's going to have, and that part is pretty much done. But this is only part of the story; the formation of eta carbides is critically important, and some studies suggest that they contribute to the wear resistance of steels more than the martensite.
Much of what happens at this temperature is due to compression. If you are studying this page, you probably know I've mentioned compression before. While the studies are complex and detailed, I'll try to put them in plain English. After the martensite is formed, expansion and compression play a role in precipitating carbide development. The martensite formed is, in simple terms, cramped, expanded, forced, bent, and strained, but the cooling continues. Due to laws of the states of physical matter, the steel is compressed by the cold. The metal shrinks in the extreme cold, pressures increase within the structure, and this physical state forces lattice deformation, shifting carbon atoms, stacking, arranging, and shuffling the crystal form. Carbides then form by the spinodal decomposition of some of the martensite.
While this can get pretty complicated very quickly, and there are great minds and works on the process at the crystalline level, the concept is this:
The point here is that the extreme cold does not slow the process in a detrimental way; the extreme cold forces the process to continue past martensite conversion on to eta carbide precipitation. Now consider this; at least one study confirms that deep cryogenic treatment for extended times (20 hours was used in the experimental process) actually increases the inherent driving force of carbide conversion that takes place in tempering. This means that there are structural changes that happen in Dcryo that enable more carbides to be formed when the blades are tempered! And this study was done on D2.
Very neat and exciting, isn't it? Okay, maybe it's a cheap thrill; it's not like the day you bought your new car or your boat, but for guys like me, it's a thrill to be alive at a time when we are learning, improving, using, and sharing all this data.
Hi Jay From South Australia
THANK YOU! Your website is a rare treasure. I have learnt more in one evening from your website in one than a month of scouring the web on blade technology.
I am and have been a part-time hobby jeweler for years who had aspirations of making a knife for myself as a display of my "talents". That is the problem with finding the best out there. It can make you feel like an infant who has just shat in his nappies! I'm confident in my design skills as a jeweler but when it comes to blades I am a noob and WAY out of my league.
I'm going to use your site as a study reference as this art is something I am determined to learn. Was trying to find someone to do a blade on commission to fit my handle but was not satisfied with what is available to me here. When reading the manufacturers (Damasteel, Sandvik, Crucible) instructions on hardening and tempering their premium martensite alloys many recommend cryo treatment for optimum tempering but have yet to find one commercial small volume heat treater here in Australia who does it. When I queried this they (the heat treating firms I made inquiries with) dismissed it as not important! HUH? So I dismissed them! Mediocrity is not inspiring. AT ALL!
That made me both frustrated and angry and also determined to find an answer. Then I come across your website and literally in one evening a lifetime of myth and bullshit on blade craft was wiped from my mind. Ahhhhhh! It was a cleansing experience I assure you!
I have a LONG journey ahead of me. Absorbing everything on your website is but the first step. THANK YOU for your honesty, your professional attitude and the dedication to your craft that inspired you to give so much. It is very and mandatory reading at a time in history when but a precious few in their given art. I wish you all the best and I am sure that better is yet to come.
Kindest regards,
Mark Gessner


What is grain, and what role does it play in steel knife blades?
The word "grain" in steel refers to the particles of the crystalline lattice, and how the word is interpreted and in what context it is used changes the definition of grain. For instance, a grain may mean the singular crystalline lattice of a microscopic particle of carbide, or it may mean the group of bonded lattices that are surrounded by another material. Grain may mean the visual appearance of finished steel or freshly broken steel, or it may mean the finest particles visible under an electron microscope.
Literally, the word grain is defined as the discrete particle or crystal determinable in the matrix. So you can see that the type of particle, the size of the particle, and even the viewing apparatus used to see the particle identifies the type of grain being described. Grain study and structure is common in steels, it can determine the material, size, shape, and bonding structure of the crystalline particles, and thus their percentage in determining the effects of various thermal treatments. There are studies about grain size, grain shape, and grain boundaries. There are studies and procedures for lapping the surface, etching the metal, and examining and counting grains under a microscope. From this, you can see that grain complexity is a science into itself, and belongs, in our case, in the realm of the metallurgist and materials scientist.
When knifemakers talk about grain, you should probably take what you read with a grain of salt. Sorry for the bad pun, but in all seriousness, grain manipulation, grain bonding, grain sizing, shape, and structure is beyond the realm of the knifemaker, no matter what forum or venue he is posting on.
This strange fascination with grain probably hearkens back to the blacksmithing or hand-forging days, when you could heat treat a piece of metal and then break it in half, and visually examine the grain. A large crystalline grain would mean it wasn't at it's best hardness, a small, fine grain meant you were close to the mark. But this is far and away from scientific grain testing and study, something I will flatly claim is out of the realm of knifemaking.
The reason this is not the knifemaker's realm is that knifemakers only control the shape of the steel blade, its geometry, and the process of heat treating and finishing of the steel, and do not control grain structure. Mistreatment and bad practices on the part of the knifemaker will result in an inferior blade performance, and some of these defects may be visible in the grain structure. Understand that no knifemaker is working under an electron microscope, and no knifemaker imparts some special magic in his process to manipulate grain changes in the steel that are improvements on standard process and cryogenic processes.
The reason I'm detailing this is that I've seen these "grain discussions" for years, on forums, in postings, on websites, and in bulletin boards, and nearly all of them are bunk. Guys are claiming that chromium carbide grains are too large to bond at a cutting edge, that grains are soft, grains are hard, grains are improperly placed. All of these discussions are meant to try to explain why their knives, their idea and interpretation, their choice of alloy is somehow superior than other choices.
No where is this more apparent than in the most persistent misrepresentation (lie) spewed in knifemaking, that somehow carbon steel blades are superior to high alloy stainless steel blades. Guys use this grain argument over and over, and in creative yet unsubstantiated ways to claim that these inferior steels are somehow superior, and they use grain discussion to bolster their argument. After all, who is really examining grain, and to what degree? Do these guys have Ph.Ds.; are they published scholars; do they have any evidence by such to prove their claims?
The next time you read some claim about grain, consider the source and challenge the source if you must, but it will be a fruitless endeavor. When a knifemaker creates a knife, he only knows what to consider to prevent disasters like grain growth during extended soaking at austenitic temperatures, and non-conversion of allotropes by not reaching or holding the steel until its martensite finish temperature. Correct processing in all steps is critical, but a knifemaker will not improve on the steel apart from the best processing procedure possible. Some things we do know about grain:
It's interesting to note that nearly always, when knife enthusiasts and knifemakers are faulting grain in knife blades for bad or inferior performance, they are writing, talking, or posting about chromium carbide. "Chromium carbides are the culprit," they claim, with "large grains that make the steel impossible to sharpen," or "chromium carbides have bad or inferior bonding to other grain particles," or "chromium carbides pull out of the steel," or "chromium carbides lead to an impossible to sharpen knife."
Knifemakers on forums claiming to be machinists even state that these carbides are soft! This is clear and obvious ignorance, as any engineering and metallurgy source will quickly and clearly prove that these carbides are extremely hard, and add tremendously to the wear resistance and durability of the alloy in a myriad of ways. This is one of the main reasons chromium is used as an alloy, after all! I'm not talking about simply adding chromium in a lower carbon steel for increased corrosion resistance; I'm talking about chromium in high carbon martensitic steels, where the desired result is chromium carbide because it is so beneficial!
A lot of makers like to cherry pick bits and pieces of data they think bolsters their argument of why chromium is bad in steel. One study suggested that chromium carbide particles were pulled out of the steel in high wear testing. Of course, this does not mean that chromium carbide was in any way less beneficial, yet makers will glom onto any bit of data they think means that the entire machine tool industry is wrong, and they are right for choosing a lower alloy to hammer into a blade. Note that in this one study, the claim was that these were sub-microscopic effects only occurring on high speed, high pressure, high temperature machine cutting tools, at sub-microscopic levels in tribological testing (the science and engineering of interacting surfaces in relative motion).
This is not in hand knives! This is because hand knives in use experience tremendously lower pressures, feed rates, temperatures and abrasive motion stresses than high speed tool steels, hard-surfaced tool steels, or machine-driven specialty steels, where tribological studies are necessary. No one is using a knife blade cutting at 200 surface feet per minute to cut a carbon steel bearing block at high feed rates and elevated temperatures. A hand knife is not a milling cutter, in other words, so these discussions bear little on knives.
Again, to understand how minute these particles are and they do not determine the ability of a knife to be sharpened and to cut, please read about carbide particle nonsense on my "Blades" page at this bookmark. It will open your eyes to these ridiculous claims and illustrate just how small grain is, and how comparatively wide the sharpest cutting edge is.
Another common ploy among the ignorant is to claim that high alloy steels in general, and stainless or high chromium steels in particular, are inferior to carbon steels made into knife blades because these high alloys are not used in particular machine tools. They cherry pick (again) to bolster their argument, by citing specific machine tools that may use a lower alloy cutter, or axle, or guide, or runner, or former, or some other component. Then they'll claim, "See? I told you that high alloys and stainless steels are bad, otherwise these machine tools would be equipped with them!"
What they don't go on to clarify, as I will here, is that the economy of manufacture prohibits the use of higher alloy steels; they are just too expensive to use in machines that are made cheaply! Most of the time, these machines are made in foreign countries, (India, China, Singapore, Taiwan, Pakistan) and other locations by firms that are not known for their use of high quality production, high quality parts, or high quality anything! They are budget-driven and volume-driven firms, not quality-driven firms. So these manufacturers opt for cheaper products overall. Even in machine tools that are not cheaply made, the use of an extremely high alloy is not often justified when a less expensive steel will do.
Another limiting factor is that the extremely high performance value may not be necessary or applicable in the range of wear or exposure. For instance why have a 440C stainless steel drive shaft on a planer that has a plain steel chain driving it? The application may not require it, and the use is not appropriate. Just because there are high alloy (and expensive) steels available, this doesn't mean that they are the best choice for all equipment or machinery that does not meet that high quality standard, so they use the most economical steels. Is this the reason why a knifemaker would hobble his clients with a lower alloy, lower performing steel?
Now think about this for a moment: would you want these cheaper, lower alloy, less wear resistant, less tough, less durable steels used to make the turbine blades for that aircraft you or your family is flying on? How about the ball bearings for the landing gear, with improved corrosion resistance? That would be (detailed by SAE and AISI, AMS 5880 standards) as the "premium aircraft quality product: 440C." It's got at least 16 percent chromium for a reason. This is not a casual hobby designation, this is the Aerospace Materials Specification (AMS) standard.
Note that these guys typically have something against chromium carbides. Why is that? Could it be that they are fans of carbon steel blades, steels that have very little chromium, and are fans of non-stainless steels in general? Are they looking for excuses to use, promote, and work with non-stainless steel blades because of their own interest and skill level, and can't admit that their blades are inferior? .
Of
course this is the reason, because if they knew anything about metallurgy at
all, they would know that chromium, the hardest metal on the periodic
table, is a wonderfully positive addition to steels, used even in low alloy
steels like 52100 (the princess of many hand-forging knives) as an addition to
improve hardness and wear resistance! Here's an interesting
detailed example of this foolishness on my "Blades" page:
Does Chromium hurt or help the blade?
The only thing a knifemaker can do is choose a steel that is the best he can afford for the project, suited to the knife project and expected exposure and use, and heat treat it with the best possible method. The high alloy and stainless high alloy martensitic steels are the best performers made for the applications of fine handmade knives, unless the knife is designed for decorative and primitive appearance with pattern-welded damascus or temper lines and rough finishes, or the knife is made with extreme economy (cheap) in mind. There is a reason high alloy hypereutectoid steels outperform all others in these applications in professional, industrial, and military use, and there is a reason that machine shops are not using blacksmith-made products in any type or circumstance. When someone is claiming grain boundary, grain interaction, grain shape, grain inadequacy in any way, he is talking nonsense.
Hi,
I can't afford one of your knives but having dabbled at making a couple for myself I just wanted to say how in awe of
your work I am....your knives are WORKS of ART!!! I can only aspire sir!
Kind regards,
Gareth Mackenzie


After reading and studying this page, the question in your head about fine knife blades should be "Why not?"
This may be the most complicated topic on the page. The reason is because people don't want to change and grow, they don't want to admit there might be a better option, they may be ignorant of science, scientific process, or metallurgy, they may want to justify their own way of doing things, or their concepts and understanding of the idea of process applications was limited. it simply may be out of financial and investment reach in the studio, as these process and equipment add to the expense of fine knife creation.
These same attitudes nurture the tired, outdated myth that carbon steels make better knife blades than high alloy stainless steels, that hand-forging is somehow better than high alloy machining and laboratory-grade heat treating with cryogenic treatment, and that a primitively-made knife has some durability value rather than decorative only. These are all past myths, and it's surprising how they are defended, propagated, and reinforced by knifemakers who should know better, and should be better educated on their metallurgy. We've got guys selling carbon steel damascus chef's knives for tens of thousands of dollars, simply because of popular television exposure, claiming the experience of every century of blacksmithing as their own, merely to justify their outdated process! Would it help to know that there is no modern machine that endures any measurable force and stress that has one single part that is hand-forged? Would it help to know that there are no blacksmiths at NASA, at any AISI member's business, at no machine shop, at no research facility? Yet these stubborn myths remain, mainly because of money and ignorance.
One scientific study in 2010 relates that while heat treating overall was well understood and employed, cryogenic treatment was still in its infancy. This is a critical point, and I'm very excited about the prospects of what this relatively new understanding of science offers for our field. Even companies who have this performed on knife blades may know little of the process, simply because new and revealing details are being discovered and uncovered every year. Though it was known back in the 1930s and 1940s that cold treatments were beneficial, it has taken decades for the details to be wrung out via studies and advanced microscopy, equipment, communication across fields, and testing, and may take many more years for it to become the standard, though I absolutely believe it will.
Unlike the company that sells milling cutters by the thousands, I strive to make the very best, and do not function by volume but by quality. This is part of the division between knifemakers as individual artists and craftsmen, and knifemakers as companies that want to make and sell a lot of knives. Realistically, I don't want to sell a knife to every person who comes to the website or hears of my name. I only want the right knife to go to the right person. That is a unique and sobering conversation that I'm lucky to have. The right knife is built in extremely high quality throughout. The performance must also be a robust as I can possibly supply, and that is the reason why cryogenic treatments have a place in my studio.
"(Cryogenic treatment) has many benefits. It not only gives dimensional stability to the material, but also improves abrasive and fatigue wear resistance and increases strength and hardness of the material. The main reasons for this improvement in properties are the complete transformation of retained austenite in to martensite and the precipitation of ultra fine eta-carbides dispersed into the tempered martensitic matrix. Numerous practical successes of cryogenic treatment and research projects have been reported worldwide. However, the treatment parameters including cooling rate, soaking temperature, soaking time, heating rate, tempering temperature and time need to be optimized with respect to the material and application.
Comparison of Effects of Cryogenic Treatment on Different Types of Steels
--P. I. Patil, R. G. Tated
2012


How does it work? Dry ice (solid carbon dioxide, CO2) is purchased and crushed, then put in a liquid that will remain liquid at the cold temperature of the dry ice, and a bath is constructed. The knife blades have been brought to their hardening temperature, and then quenched to room temperature, and then are dropped in the very cold bath to complete the quenching.
It's pretty important how the bath is made, chilled, and stored, as are the components of the bath. By the way, these baths are fairly common in use for benchtop laboratory study and process, when a very small amount of cooling is needed, and the lab does not have the funds or resources to purchase a dedicated mechanical chiller with a circulatory bath.
Sounds simple enough, and it has its place, but also has some pretty significant limitations that the knifemaker needs to know and the knife client needs to be made aware of, particularly since there are significant factors that may result in a less than optimum knife blade. While dry ice bath quenching is an improvement over conventional heat treat, it is not the pinnacle of quenching methods and in this section I'll detail the process, advantages, and limitations.
Dry ice is very cold; it sublimates (turns into gas) at −109.3 °F (−78.5 °C). If you can get it in a saturated solution of liquid, that solution will be at a very cold temperature, close to the shallow cryogenic process temperature. Put the knife blades in the solution after quenching to room temp and let them get really cold. Sounds great, but there are some limitations.
In the old and early days, we used acetone for the bath medium, but this is not a good choice due to its instability, flammability, and reaction potential with nearly all plastics. Acetone is a solvent that loves to dissolve things, so other solvents should be used. One of them is ethylene glycol, but this is poisonous, and it's probably not a good idea to keep a volume of it around. Ethanol is another bath solvent, but it's highly drinkable and might disappear when you have visitors in the shop that appreciate fine liquors or Everclear® (just kidding). Denatured alcohol is a common enough agent, though it evaporates quickly at room temp and is flammable so storage has to be carefully considered. Most shops don't have a flammable safety cabinet, and this is the proper way to store this type of agent.
You might read that dry ice sublimates at −109.3 °F (−78.5 °C), so it might be considered that this is the temperature of the bath, but this varies greatly. The dry ice can actually be much colder, depending on how it is made, where it comes from, how it is stored, and how it is transferred. The liquid in the bath may be warmer, unless continuous agitation is employed. So the temperature can vary quite a bit, so to keep accurate records of process, a thermocouple should be employed to determine the temperature of the bath.
There are several substantial issues with using dry ice baths for quenching. While they are a step in the right direction, they are not optimum for quenching steel knife blades and have substantial limitations for these reasons:
What to do? While dry ice baths are a step in the right direction, and they are better than conventional heat treating (CHT), there are better methods, and this is why I don't use or recommend dry ice baths for the ultimate treatment of hypereutectoid and high alloy stainless tool steels.
However, if a maker is determined to use a dry ice bath for quenching, he can attempt to overcome some of these limitations listed above, and then he should be able to explain these to his clients:
If you, as a knife client, customer, or user, now think that dry ice baths are not the most efficient way to quench steel knife blades, congratulations. This is something I learned years ago before I moved to other methods and equipment. Remember, dry ice baths are a step in the right direction, and they are better than conventional heat treating (CHT), but there are better and more reliable methods. Because my clients deserve the very best steel treatment possible for their blades, this is why I don't use or recommend dry ice baths for the ultimate treatment of hypereutectoid and high alloy stainless tool steels.
Hello Jay,
I recently got into knife making as a hobby. I came across your website and I just have to say you make
some of the most Beautiful and Functional knives I have ever seen. It is wonderful to see such things as
inspiration to always strive for better quality.
That's pretty much all I have to say, but I felt I had to let you know how amazing I think your work is.
Sincerely,
Sean Mckernan


I've described the role that cryogenics plays in the quenching process of knife blade steels, and how the deep dive and hold into the realms of cold has a positive result and improvement of steels, particularly high alloy steels used in the finest knife blades.
There are companies that sell a service of post-heat treatment cryogenic exposure or immersion. They call it "Cryogenic Processing," "Cryogenic Treatment," and even "Cryogenic Tempering," which are all very confusing terms, as the industry has no standard process definition for what they are doing. If you do some reading into the service they are selling, you'll see that they are offering a post-hardening, post-tempering immersion into either liquid nitrogen, nitrogen gas, or nitrogen spray and a cold cryogenic soak. Are these viable services, and do they help the steel, particularly knife blade steels? It's important to understand where and when this process takes place in the tool creation, and ask some serious questions about why this is considered.
The general sales pitch is this: send us your completed tools and steels, already hardened and tempered, and we'll simply run them through the liquid nitrogen and this will improve the wear resistance, toughness, hardness, etc. That's it. The steel is already hardened and tempered, and at some indeterminate point in the future, you simply get it very cold and hold it there for a while, and the wear resistance is somehow improved.
If this appears a bit questionable to you, I understand and concur. Considering where in the process cryogenics plays a role (in quenching and before tempering), this seems to me also to be an empty promise, particularly when some of these companies cite the statistics of increased wear resistance that occur during cryogenic quenching as being available in their process! I'll flatly claim that this is misleading at best, and a lie at worst. Perhaps they are just ignorant of what happens during actual cryogenic quenching process, but I don't know why, since the results are extremely well documented. Conversely, I can find no studies bearing any important or substantial result of cryogenic processing after hardening and tempering that improves the steel in any way. If there were proof, this would be standard process for all steels, in all tools, and it is not. We would be digging out all our old cutters, chisels, drill bits, metal forming dies, shear blades, and every tool steel item we could find and dragging it through liquid nitrogen to improve it, and we don't. We don't because this is not a proven improvement to the steel, and therefor it is not the standard. The standard and premium process is cryogenic treatment during and as a part of quench, not post tempering! Where is the actual tribological testing to prove the claims of these post-tempering process improvements?
Perhaps the reason for this is that once complete tempering cycles are done, the steel is about as stable as it's going to get. The conversions have taken place, the thermal cycling is done, the steel allotropes have stabilized. While it's possible that some retained austenite may be converted in steels that have inadequate martensite conversion, it's unlikely that the amount is significant to realize any noticeable improvement in wear resistance after tempering.
I've done my own studies and experiments and concluded that once a knife blade is hardened and tempered, at any indeterminate point after that, simply getting it very cold does not markedly change the hardness and performance of the blade. I haven't tried this with all steels, just the most predominant types I use for knife blades, and I've seen no reason whatever to do this.
Of course, there are many types of steels and applications, and a large amount of variables that have to be tested to confirm a viable advantage to post-tempering cryogenics. When considering the heavy sales pitches and promises, I would like to see some viable scientific studies done on the actual steel items treated. While cryogenic processing during quenching is extremely well documented, post-tempering cryogenic exposure is not. Could it be that some companies are simply selling a service that is related to quench process in terminology only, not backed by proof? Where is the actual tribological testing to prove the claims of these post-tempering process improvements? I'll leave that up to you to consider. In my studio, cryogenic processing is part of quenching, not some afterthought that is somehow supposed to change an already stabilized and tempered steel.
I'm only writing about high alloy hypereutectoid and stainless steels, not brass, aluminum, castings, or nylons. I'm not writing about plastics, tissue or food products either, so if you are one of those companies performing this "treatment," please be kind enough to cite studies that prove your claims. Unfortunately, there are few references included in the advertisements for these processes, even though documentation is fairly accessible and common these days.
Now for the few companies that request that you send them hardened and quenched steel items that have not been tempered for them to cryogenically treat and then temper them, it's critical to remember that timing is key in this process. The longer the steel exists in a quenched and untempered condition, the much greater the risk of dimensional instability and stress, leading to micro fractures that may reveal themselves as high wear, even after tempering! Martensite can not sit at room temperature; it is metastable until fully tempered! Add to that the steel isn't at full stability until several thermal cycling operations. Full quenching and tempering must take place immediately for these high alloy steels. There is no time to wait, to ship, to get in line for some distant company to run them through a cryogenic process and then temper them at their convenience.


Most manufacturers and foundries have white papers with clear illustrations, charts, and comparisons available, for free, available online. They are also called data sheets and other descriptive terms. Beware, though, that these are advertising documents, and some of the claims can be outright misleading, so some interpretive logic has to be applied by the knifemaker or knife enthusiast seeking a particular steel.
For instance, one supplier claims that their steel has the same or better corrosion resistance than 440C, but that is a gross generalization, as it doesn't even indicate the condition of the 440C. The steel this supplier is selling can not be mirror polished, so that is a huge factor in a steel's corrosion resistance, detailed here. Another factor is that the hardness of the 440C is not specified, and the corrosion resistance of 440C directly corresponds to the hardness of the finished steel. Still another consideration not identified is that steel corrosion resistance can vary depending on how they are quenched and treated!
These documents often feature comparison graphs (more about comparagraphs here), but the graphs may not specify ranges or details of the comparison. So you might see a bar graph with bars of the advertised steel towering far above the bars of a comparative steel (typically 440C, since it is the standard others are compared to for a reason).Be aware of these types of graphs circulating on the internet, without specifics of how steels are compared. Note that in every single case, the steel being peddled is the one excelling in all relative characteristics above others (shocking, no?), and the testing method and treatments are not even specified. More on knife blade testing here.
These advertising documents also may not specify what happens or what is expected in cryogenic treatment. They may mention cold treatment, or freezing, or refrigeration, but typically with little information about the duration, temperature, and rates necessary for an expected result. I've contacted engineers and metallurgists with some of these companies before, and after long discussions, it's clear that extreme testing with scientific method and in substantial lots has not been done on many new steels. This is because, according to them, most steel manufacturing is done overseas, and our colleges and universities (who used to be supplied grants to do extensive experimental testing) are suffering from the same funding limitations as businesses are. The manufacturers relied upon research institutions for the testing and development, and that's not being funded, so many questions are simply unanswered. It then becomes the province of the business experimenter to do all the research and testing, and as a dedicated knifemaker and businessman, I do my own bit of testing in the cryogenic field, with my results being field tested in some of the most demanding occupations and uses in the world. It's an honor to do this, and I'm committed, as all makers should be, to the furthering of their trade, craft, skills, and art.
If properly followed, the results a knifemaker or knife manufacturer experiences will align with those posted on the white paper or data sheet, with refinements and enhancements of the process recorded in the knifemaker's own data set. These guides are proofs and resources on their own, and worth their weight in gold to the researcher and materials developer as well as the knifemaker. They also define the knifemaker's own proof of concepts and skill, with pitfalls and errors in processing avoided and successful results and process expected over the range of steels he uses.


It's critical to understand the importance of hardness testing, not just after the heat treatment process, but all along the way. This is the only practical, frequently referenced, reliable, and logical instrument designed and used to determine correct process of proper and effective heat treating of knife blade steels.
Hardness testing may take place at many times, particularly in establishing a baseline of process if cryogenic treatments, multiple tempers, and various aging and timing steps are used. These baselines, carefully recorded and evaluated, give the knifemaker a greater and specific understanding of each steel he uses. The hardness tester plays a large role in these process adjustments, as does the heat treater's log and record. Though the data sheets and white papers for each steel give an idea of hardness and results, these are only a rough guide, and I've found throughout the years that results can vary tremendously from these supplier-side references. The engineers and metallurgists at the steel company will assure you that results vary greatly from their data sheets, and that the only way to be certain of each item is extensive testing, comparisons, and evaluations of each item processed. The only way to be sure of the individual knife treatment regimes is to accurately and frequently test each knife along the way. This also assures the knives fit the maximum performance criteria that the high-tech alloys are designed for.
Testing can and, depending on the method, should occur before heat treating, after the initial quench, after a shallow or deep or cryogenic processing, and before and after each temper, if necessary. This is a lot of results and data, and can give the maker tremendous insight into how, when, and thus, why changes are occurring in the allotropes and crystalline structure. For example, it's critical to know that 440C, which is not known for supplemental hardening after the first temper in any reference, can actually experience this if the maker is pushing his steel process into a refined and extensive custom procedure. Testing can also indicate the important tempering times and temperatures for desired results. From my own experience, I can assure you that the data sheets are not accurate, and typically, no manufacturer or foundry is experimenting with high process control to achieve improved response, due to the reasons listed in the topic "Why Cryogenic Processing?" on this page.
In my studio, I typically use the hardness tester pictured below. How the device works: The blade is placed on the anvil, and tension is adjusted to relieve play. The device uses a specially ground diamond-tipped penetrator for high hardness testing, and this diamond must be regularly checked and examined under a microscope to assure its integrity and geometry. Other penetrators (tungsten carbide) are used for softer metals and lower scales of hardness. A 10kg (22 lbs.) load is first applied, which causes initial penetration. The major load is then smoothly and accurately applied. In the case of a diamond penetrator, at this high hardness range, I use a 150 kg (330 lb. load), and penetration occurs. That's quite a bit of pressure on the tiny diamond, so the integrity of the diamond, the mounts, the anvil, threads, bearings, linkages, and dashpot that regulates the rate of pressure application must be smooth, clean, and frequently checked, serviced and tested for accuracy. The penetrator only penetrates so far, and the penetration stops. The major load (150 kg.) is removed, with the minor load (10 kg.) still applied, and the penetration is measured. This gives an accurate reading of the exact hardness at the point of penetration, and thus the relative hardness and temper of the whole blade. The testing mark is usually placed where it won't be seen, since the mark is permanent in the blade.
HRC: You'll see this initialism (not abbreviation) frequently if you look at a lot of knives. Simply put, it means Hardness, Rockwell, C (scale). There are many ways to measure the hardness of metals and other materials, but in knives, we are mostly concerned with the blade hardness. Knife blades are tools, and cutting tools are measured on the highest hardness scales of the Rockwell tester, a penetration testing device. There are many scales in Rockwell hardness testing, A - V. These scales vary depending on the material being tested, and the hardness tester uses various penetrating styluses, and varying pressures applied to test them. In knives, you'll generally see two scales used; Rockwell C (HRC), and Rockwell B (HRB). If a blade is measured in HRB, it's markedly softer than HRC. Sometimes, knife manufacturers will give a hardness, and it might appear high, but somewhere in their text, they'll identify that they are using the "B" scale. This is a tricky, misleading feint to get the customer to think a knife blade is harder than it is. On one "evaluation" of chef's knives, I read the hardness represented as "HCR." This is the sign of a sloppy amateur writer, or an unknowledgeable knife enthusiast.
Though the hardness tester is a fairly common sight in complete machine shops, it is sometimes neglected. This is a fine instrument, capable of very accurate readings when properly maintained and used. The delineation of this machine is close to a millionth of an inch, so any error is significant! For example, a slight deviation of the penetrator by the compression of a dust in the bearing surfaces of the anvil that leads to one millionth of an inch displacement can cause significant error of the final reading. This is a delicate instrument and has to be regularly cleaned, calibrated, and maintained.
One more important hardness testing consideration: any malformation, tiny microscopic irregularity, minuscule flaw of, around, or on the diamond penetrator will give inaccurate results. I've seen makers reveal that they have impossibly high hardnesses, and I suspect this is the error. If the penetrator has a chip in it from careless or sudden load application, angled application, or general wear, it will give a reading much higher than the hardness actually is. This is because the carefully ground geometry of the diamond is critical to accuracy. A chipped or worn diamond will tell you a knife blade is 67HRC, when it's actually much lower. The diamond can't penetrate as much, so the instrument gives an inaccurate reading. You'll need a microscope to examine the diamond for flaws, at least 30 power is usually sufficient. More reasons to have the machine regularly calibrated and tested against standards, and the measured hardness of the blades tested on an alternate instrument, by another lab, firm, or entity. There are other issues with hardness testing and these instruments that I may go into in my book.
The condition of the steel blade or item to be tested is very important. Steels with a rough finish do not test accurately. Imagine trying to establish a standard measurement of penetration in a surface with a bunch of lines (grind abrasive marks), irregularities, or contaminants on the surface. The steel surface to be tested must be very clean, smooth, and clear of debris, inclusions, or contaminants.
I've read where hardness testing is done on a fire-scale surface, and this is an amateur and glaring mistake. To get an accurate reading, the maker must be reading the steel surface that is the final finished surface that the knife will have, essentially the core of the steel. This can only be reached after heat treating by removing perhaps .0003" to .0005" (half a thousandth of an inch) or more of the surface. This is significant grinding, and I've read where outside heat treating companies who are not keen on grinding on someone else's knife blade surface simply take a Cratex® (rubber impregnated with silicon carbide) wheel and clean off a tiny spot to do the testing. This is a minimum of consideration, and perhaps more cosmetic than accurate. Spot abrading does not produce a highly accurate and valid test, and the reasons are several.
Another factor in establishing an accurate reading is the degree to which the surface is finished. Some ASTM standards require "lapped and polished" specimens! None of this "grind the spot to 400 grit and you're done." Remember, this is in the final test that is done, the number that will accompany the knife. While in my experience the degree of finish can vary the final reading at up to one point depending on the finish, in this world one point is significant variation. Standards also recommend that the underside of the tested piece is also finished, as any microscopic movement or deviation against the anvil (think sliding or slight displacement or compression) will affect the measurement.
The surfaces to be measured must be parallel, testing surface to anvil contact surface. This seems obvious, but an angled surface will cause sideways pressure as the geometry becomes a wedge which will effect the reading as pressure is applied.
Thickness must be considered; a blade that is 1mm (.040") is too thin to test in the standard Rockwell tester. This is not often seen but on thin chef's or fillet knives, but is also a reason that the blade is not tested in a thin region (near the cutting edge or on thin tangs).
Testing must occur away from any edge (or hole or any irregularity). It stands to reason that since the metal is deformed, if the test is near the edge of the material, it will be deformed at an angle, and the reading will not be accurate.
Time is seldom considered, but did you know there are standards as to how long the pressure is maintained at the contact point of the penetrator? This is because steel deforms plastically, even very hard steel, and it may take minutes for this to occur.
All of these reasons add to the logic of a maker testing his own knife blades. The tests should be standard, an average of multiple tests, and the maker can control all of those tiny yet significant factors that contribute to the viability of the tests. Farming out knife blades for heat treating will not establish these practices, an outside heat treater does not get paid to experiment or vary process, or test along the way, or establish standards in his own testing regime. This may be well and good for the hobbyist knifemaker, but for the professional, a higher standard of measure is desired by both the maker and knife owner.
I'll go into this in greater detail in my book, but I've included it here to help the reader understand just what is required in establishing an accurate guideline and framework of testing for knife blade hardness to aid in processing the steel to its highest performance, and establishing accurate benchmarks for the client and knife owner and user.


For complete transparency, please note that since my first knife made in the 1970s, until the present day, I've heat treated every single one to the best of my knowledge and ability. Know, also, that I've never had one failure, not one return, not one complaint about the hardness and wear resistance of a single knife blade I've made.
Heat treating is not mystical wisdom, not a mystery of scientific knowledge, and not an unobtainable goal: it is simply a process. It's hot, it's cold, it's timing, it's workflow. It's numbers, it's temperatures, it's logical, like any process. And like any process, understanding, control, record, and repeatability are the keys for reliable results.
In this section, I use the word outside because this refers to people who heat treat outside of the premises of the knifemaker's or knife manufacturer's own studio or shop.
There is quite a bit of money to be made by contracting heat treating, with very little relative expense. Once the equipment is paid for, it's only electricity and expendables (mainly liquid nitrogen and gases) to pay for, and the business can be quite lucrative in large batches. This is because there are many people and companies who make knives (and other cutting tools) and few who understand or who can perform the process of proper heat treating and processing. More about this in the Equipment topic below.
There is nothing wrong with outside contractors, particularly if the maker can not reliably heat treat his own work. But us older guys who see this in the trade know that if the maker doesn't heat treat the blades himself, he's handicapped and limited in the knowledge and scope of his work, and is simply not in charge of the entire process of making a knife. I'm sure I'll get some hate mail over this, as guys frequently write to me to justify what they are doing and how it's the best, most proper, most reasonable way to do things, and it may well be, at least for them.
If he's in the business of knifemaking and calls himself a professional, his clients simply hold him to a higher standard, one that assures he knows all of the particulars of the field, and steel work is the entire scope of the blade. If he doesn't perform all of the necessary operations of the blade making, how will his client know that his blades are worth their salt? Will they then question his ability to interface a permanent handle with the blade, create and install the fittings, the handle material, and the sheath? Every part of this operation that is contracted or farmed out means that other hands are involved, other ideas, other skill levels, other process and procedures, and other levels of quality are in play.
Heat treating is the foundation of knifemaking.
This concept of contracted outside services help can then be logically extended. Since it's easier to send a blade out for someone else to heat treat, it's clear to the client that the maker is interested in the easier way. Most guys won't get this, but understand: IF A KNIFEMAKER DOES THIS, his potential clients will know that he is taking an easier step, and will wonder what other easier steps he may be taking. This will affect his reputation and standing as a knifemaker, and it's been demonstrated over and again in this field.
A manufacturer or maker contracts out his heat treating, it's cheaper and easier. He may then contract out his blade construction, logically to foreign shops (in India, Pakistan, Taiwan, or China) because it's cheaper and easier. He contracts out the handle supplier, and the sheaths, all the while making things easier and cheaper. Reasonably, he's then competing with all the other makers and manufacturers who do this, and the clients know he's making easier and cheaper, so they will only pay easier and cheaper prices for his work. This continues a downward spiral called "lowballing," where the only option is to make a knife easier and cheaper. That way, you can compete with easier and cheaper knife sources, but that eventually means moving all of your operations to a foreign company because, here in the United States of America, we have extremely high median annual earnings, and people don't want to work for cheap, yet most people buy cheaper and easier. This is particularly true of the majority of knife interests (and represented in our national trade deficit!) and that is why most of the knife information, companies, exchange, forums, websites, postings, and conversation is focused on the low-end market of cheaper and easier mass manufactured knives, who inevitably, contract their work to offshore labor.
Cheaper and easier is fine, if that's the direction one wishes to go, but know this; it becomes a business based on cheaper and easier volume, and this is not how fine works are created. Worse, this is not how fine works are purchased by those who seek them out.
For the sake of argument, let's say that a maker is not interested in fine works, owning and understanding the whole process, or meaningfully contributing to the art, craft, and science of knifemaking. That's fine, but don't expect him to create a knife better than any other mass-marketed product, and don't expect to be successful at it, unless he's building a company about cheaper and easier. Also, don't compare cheaper and easier knives to those that are completely made, in house, by a professional, because the two are in different leagues altogether. And don't expect a cheaper and easier knife to ever be of value to a client, as it will end up a poor performer both in the physical sense, and in the investment sense.
Simply put, if a knifemaker starts contracting out for the sake of expense, there is no logical stopping point, and I've seen it over and again. A knifemaker makes knives, and that means he makes the blades.
Every maker who uses an outside heat treating contractor swears they are the best ever! If you don't believe this, I challenge you to find one maker who complains about the result of their chosen heat treating contractor. After all, this would be detrimental to the sales of their knives, wouldn't it? It's always been curious to me that every single knifemaker crows about the results of their chosen heat treating contractor's results, no matter who they are. Are there any realistic comparisons of each contractor's process or results? Does the knifemaker even know or understand the process and timing of the procedure, since different procedures (like snap tempering) produces lesser results? Or, as knifemakers, are they simply taking what they get and then using the heat treating contractor's name to bolster their own knives value and performance expectations? Perhaps, just perhaps, they can justify why they don't do the heat treating themselves, because, after all, they could never be as good as old (insert name here)'s heat treating process...
It was shocking to read in a forum posting about a knife blade that was treated by an outside contractor, and the maker then had to deal with rounded moons of different appearance of the steel on the cutting edge and spine of the blade. These "blemishes" were there after grinding and sanding, and the maker wondered if there was something wrong with the steel. Then, another maker who used the same heat treater chimed in, claiming that the marks were there from the "torch process" that the heat treater used to straighten the blade! What? This is an atrocious screw up on the heat treater's part; no torch should ever be used to straighten a blade; hell, no torch should even be in the same room with a knife that's been heat treated! This is a ruined blade, a blade where localized heating has completely changed the integrity, crystalline structure, and the entire allotrope of the steel in spotty locations, and that is why it looks different. It's botched, it's ruined, it's garbage and unlikely to be saved, and the guys discussing it shrug it off as it it's something that's normal and routinely done! How terribly sad, not for the maker (who is plainly ignorant of the process) or the guys who think it's normal (who are plainly ignorant of the process), but mostly for the guy who buys the knife and finds that it has soft spots, or high wear areas, or reduced toughness in spotty locations, or finds that the blade eventually cracks from dimensional instability. Yet the heat treating contractor goes on to treat blades this way as if he is doing the right thing, and the guys who use his services claim he's the best... truly sad.
What if the heat treater screws up your blade? What will they do? You'll have to get a clear contract from each individual business to find out. Most of them will give you a value (coupon or money or purchase potential) for the size of the blade in raw stock. Yep, you get another bar of steel, raw, so you can start over. This may be fine for some makers, but it's not how I would want to do business. After all, the value in the blade is in the labor that has (hopefully) gone into the blade before heat treat. That may mean profiling, drilling, grinding, filework and in some cases, even engraving! Yikes; that could be many, many hours and that's a hellofa hit! As a maker improves his work, this can be a substantial amount of the value in the knife, and I'll flatly claim that it's usually many times more than the value of the raw steel. But if a heat treater screws up, a bar of raw steel will be all you'll get for your trouble. That is, if he admits he made a mistake at all! I disclose this because typically the heat treater will blame the maker: you've ground it too thin, you've ground it unevenly, you've got the wrong type of steel, or other reasons that a blade may be "less than optimum" after heat treat. By the way, less than optimum means warped, cracked, bent, curved, wavy, or damaged.
Heat treating overall is a fairly simple and straightforward process, clearly outlined in the manufacturer's white pages and through endless online sources. This is another reason to do these operations in the knifemaker's own shop or studio, so the maker himself is responsible for the very best treatment of knife blade throughout the entire process, without delay, with complete control of the process and thus the results. This is why many of us old-timers claim that if you don't heat treat your own blades, you're not really a knifemaker.
In the decades I've been doing this (full time professionally since 1988), I've heard a continuous, unsolvable argument about heat treating by the knifemaker, and farming out this process step to others. It's sad to see that it has caused such grief and embarrassment, complaint and conflict among makers, and here's how it goes:
A guy wants to get into knives. He makes some with simple hand tools and then quickly realizes he needs more equipment. He gets what equipment he can as time goes on, and the improvement and enjoyment of his craft builds. This is how it should be. I don't know of any maker who was gifted an entire functioning shop or studio, all at once. So the modern knifemaker is then building his skill set, his tool set, his process understanding, and the result of this should be obvious and apparent in his works, his completed knives, sheaths, stands, cases, and accessories.
I'm different than most guys, I suppose. I didn't get into making knives because I wanted to make and have knives; I got into this because I was fascinated by the process of heat treating. That you could create such wide and variable ranges of steel hardness, toughness, flexibility, corrosion resistance, and appearance of steel is what captured my interest. As I stated in my bio, an old welder told me if I wanted to understand heat treating, then make a knife. The reason he knew this is because a knife blade is truly a special case. It's not structural steel that must support a piece of equipment or building, and it's not tooling steel that must be made to its absolute highest hardness for extended wear resistance at high feed rates. Knives are special, they have to be hard and tough, wear resistant and tenacious, a bit flexible, and (in most cases) corrosion resistant.
I started with a torch; a #12 rosebud (heating tip) of an oxyacetylene rig. I quickly learned that the process with this rather crude tool was tenuous at best, and highly uneven, but it was all I could afford. It didn't take long to come up with the resources to buy my first burnout oven, because at the time I started, there were no dedicated knifemaker heat treat furnaces. I modified the oven by doubling the element size and modifying the controller, to make it into a rapid ramp furnace. I then built a furnace in a discarded refrigerator body, and it had an internal furnace and internal quenching chamber, was evacuated by vacuum pump and infused with dry nitrogen! This was a real beast, it could gain 500 degrees F a minute when empty! I progressed beyond that, and am still progressing.
My point is that I've always believed that the heat treatment of steel was the basis of knife making. The very basis. It's what differentiates the knife blade from the raw stock and separates the cold chisel from a scalpel; it is the very foundation of why a knife is a knife and not just a piece of metal with a cutting edge on it. Most makers (and clients) agree.
There are those who, for whatever reason, do not wish to do their own heat treating. I know why: it's too expensive, it takes to much room, too much equipment, too much effort. They may not be confident in their capabilities, they don't understand the process, they simply may not be able to afford it or justify the expense of the equipment. I understand; I've stated I can barely afford my own shop, and this is how most creative artists and fine craftsmen work! It's okay to have knives treated by someone else, as long as it's disclosed, and as long as the knifemaker can explain what heat treating is and how it works for his clients. Most clients don't want to know the intricacies of carbide nucleation and propagation in evolving crystalline bodies; that's the knifemaker's realm.
Most artists, craftsmen, and creative people would prefer to have as much control of the process as is possible, particularly involving the core of what it is they are making or creating. For a knifemaker, the very core of his work is the performance of the blade which is established by steel choice, steel geometry, and steel heat treating. All of these are the responsibility of the maker of the knife.
Frequently, I've seen makers become highly defensive about this topic. Old guys like me might claim that you're not really a knifemaker if you don't do your own heat treat, and the sparks fly! You'll hear or read the argument of the absolute purist, which seems to be a go-to sarcastic and defensive posture based on this principle: "If you don't dig your own ore out of the ground, smelt it yourself, then you aren't a knifemaker either. Take that, you heat treating knifemakers!"
What an embarrassing comment, trying to compare mining with knifemaking. That's like saying to the fine artist painter that unless he shovels the titanium bearing ore out of his own mine (remember, to the absolute purist everything must originate by the hands of the maker), then he cannot call himself a fine artist. Perhaps Michelangelo wasn't a real sculptor, because he didn't hoist his own marble from the quarry by himself. A jeweler could not call himself a jeweler because he didn't mine his own gold bearing quartz. See how this goes? So the absolute purist shovels this cynical argument back on the maker who does his own heat treat, as if heat treating your own knives is somehow wrong!
This is an argument of building or creating raw stock vs. building or creating a knife. In insulting comparison, the absolute purist claims that unless you build your own raw stock, you cannot call yourself a knifemaker, thus equating building raw stock to building a knife. Of course, no one believes this, so the guy making this argument has just excused himself from heat treating because, in his mind, the two are equivalent!
But the comparison and technique is based in total falsehood. Let's get this clear. This is not a discussion about heat treatment; this has somehow become a comparative evaluation of raw stock production vs. treatment of a machined, forged, and/or hand-worked piece of raw stock and the two are of totally different operations, concepts, and even different fields! Yes, mining is a different field than knifemaking, foundry creation of steel is not knifemaking, but heat treating is knifemaking! If you don't believe so, then just quit heat treating your blades altogether, after all, it's not part of knifemaking, right?
Guys will go on to claim that cutting out a blade is the same thing. Have someone else cut out your blades, after all, it doesn't affect the "quality" of the knife. Why stop there? Why not have someone else grind them, shape them, sand them, polish them? Why stop there? Why not have someone else attach a handle, sand and finish the handle, polish and embellish the knife? Why stop there? You are still calling yourself a knifemaker, right? Why not have someone else make the sheath, make a stand, and sharpen the knife? Why not just have every single facet farmed out to different companies, people, organizations, and groups, in many different countries, and have the knives delivered to your door, and then call yourself a knifemaker? Why not?
There is no clear definition here of what constitutes a "knifemaker," specifically, exactly, and repeatedly. There is no designation of "knifemaker" on the United States Government's IRS Principle Business or Professional Activity Code which is how this business is classified by our government. So, really, who defines what is a knifemaker? You could bake apples at a travelling renaissance fair and call yourself a knifemaker! You could glean old newspaper from the county dump and call yourself a knifemaker! Why not? Where is the line? There is none, really, unless some authority decides to make one. So, go ahead, call yourself a knifemaker, no matter what you do... right?
This, then, becomes a discussion of integrity. That's a powerful word, right? It opens up all sorts of concepts like morality, virtue, reason, and truth. Man, those are those tough words that are borne out through years of service, concepts that involve others. I'm not saying that if you don't heat treat your own knives, you don't have integrity. I will say that if you make the base comparison that raw stock manufacture is the same as heat treating a knife blade for the purposes of justifying why you don't heat treat, you don't have integrity.
Then there is the work scope argument: I've seen these guys take it a step further, claiming that unless you're a professional heat treater, you can't treat your own knives adequately! That's the ultimate in farming-out justification.
Okay, let's accept that premise just for fun. This means that you can't drill an adequate hole in a knife blade, because you're not a machinist. A typical machinist has completed a certified, often regulated level of training or apprenticeship recognized by an official governmental organization. The machinist fixes the work to a trammed table on a dedicated machine, centers his hole using a centering indicator, or digital readout, from blueprints, and starts with a spotting drill progressing to a combination drill/countersink and then follows with screw machine drills, larger and larger drills, followed by a drill just slightly smaller than the final required hole size, and then he reams the hole with a chucking reamer, and then measures it with a calibrated pin gauge. A knifemaker therefor can't drill his own hole; he doesn't drill professionally; he's not a machinist! By the way, ask a machinist try to grind a knife offhand, and he'll tell you to go to hell, because he's not a knifemaker!
More fun: a knifemaker can't possibly fit a handle to his blade, because he's not a carpenter. The carpenter would carefully store the wood, making sure that the moisture content is correct, using dedicated tools to plane the raw stock and determine the specific grain arrangement desired. He then profiles out the stock, kiln-dries or kiln-ages the stock to the specific required condition, and then may hand-plane it and hand-scrape it in just the right direction and orientation for the application. He may use special chemical stains that react with the tannin in the wood to create the appearance he desires, then after this chemical treatment, uses stabilizers to stop any further reaction. Then, he starts sanding... a knifemaker could not possibly do this; it's not in his scope.
On the other hand, is a knifemaker who claims you're not really a knifemaker unless you heat treat your own knives a purist? Perhaps we can compare this to other trades, say, a jeweler. A jeweler may not mine his own ore, but he does his own cutting, fitting, soldering, and casting. A woodworker or carpenter may not grow his own trees, but does all of the cutting, shaping, fitting, bending, and treatment for surface and appearance. The potter or ceramics artist may not dig his own clay, but he does all his own shaping, forming, and firing.
Reverting back to the absolute purist, he may argue that some jewelers do not do their own casting, that some ceramics artists do not do their own firing, and that some parts can be farmed out! Do you see how this argument festers in unsolvable circles?
Here is the really important thing: most artists, craftsmen, and creative people would prefer to have as much control of the process as is possible, particularly involving the core of what it is they are making or creating. For a knifemaker, the very core of his work is the performance of the blade (after all, he's not a letter opener maker or a spoon maker). The performance of the blade is established by steel choice, steel geometry, and steel heat treating, and all of these are the responsibility of the maker of the knife. If you don't think so, ask a client this: "Who is responsible if one of these factors fail in a knife they purchase?" For instance, if the heat treating is missed and the blade is soft or has soft spots, or cracks from dimensional instability, you won't get away with telling the client that it's the heat treater's fault. More importantly, If the quality of a sharpened piece of steel even matters, why wouldn't the maker want to control this essential step? Why is it then that people take the stand of the absolute purist (if you don't dig your own ore, you're not a knifemaker) as a moral equivalent to justify why they don't heat treat their own blades?
It's because they want to be called knifemakers, that's why. If the name "knifemaker" did not matter, why not just assemble kits and call yourself a knifemaker? Why not buy the entire knife assembled and created overseas in a boxed form (these are available) and glue on a piece of wood and sand it, and then call yourself a knifemaker? Why not just buy the knife already completely assembled and then just etch your name on it and call yourself a knifemaker? Don't laugh, I've had people ask for this! You can easily see the progression of logic, and it's all based on the fact that the word, "knifemaker" means something, and means a lot!
To argue these concepts are a baseless, useless endeavor. A guy who doesn't want to heat treat his own blades will not change; the guy who believes that knifemakers should heat treat their own blades will not change. It's all about integrity of the individual. If you do it, explain why; if you don't do it, explain why: that's all the client wants to know. The client will make and understand the distinctions between the two. And if you feel some discomfort in telling a client that you don't heat treat your own blades, you probably need to examine that a bit more and make some adjustments in your process, abilities, and knives. That way, your client will be confident in your abilities and understanding of what it takes to make a knife.
Look, it's okay to have blades heat treated by someone else, particularly if if the maker can't afford the equipment, space, time, and effort of doing this critical operation himself. But in doing so, let's hope the knifemaker doesn't simply rely solely upon the heat treater's name in the trade. Let's hope the maker wants to educate himself on the process, and start heat treating because that is the core of working with knife blades and the foundation of building a good knife.
On one of the forums, an inexperienced knifemaker was shocked to find that his steel blade had a huge, rounded fracture in the spine, only discovered when he was grinding it. It became clear when he removed the outer "crust," which was, I suppose, carbon that had migrated to the surface in decarburization, which should never be there in the first place. However, the decarburization failure was not the main issue. It was clear that he was a beginner or novice, because he asked if the process of grinding would have caused the large crack. He explained that he had the knife blade treated by an outside heat treater, so he suspected his grinding technique, or perhaps the steel type as causing the fault, since "everybody" was using this heat treating contractor.
Let me be crystal clear here, so clients, knife enthusiasts, knife owners and users, knifemakers, and readers can understand this, as this is a great educational moment. No grinding technique or operation with abrasive belts can cause fractures.
Abrasive belts simply sand and grind (abrade) the surface, and unless you somehow pinch the blade between the belt, contact wheel, and frame of the machine or tool rest, no impact, no force occurs that can damage a properly-treated blade. Even if intense localized heat was created in the steel, this would never cause a fracture, it would just overheat the blade leading to other issues, like loss of temper.
In the discussion, the steel type the guy was using was immediately suspect by the other forum posters, and this was also the wrong path for discovery. No matter what knife blade steel is used, grinding would never cause a fracture. Add to this, the steel he was using was Nitro V, a distinctly low alloy, low carbon stainless steel that has the main advantage of being "easy to grind" and forgiving (and ductile), and a fracture seems absolutely incongruous. The posters kept blaming the steel, over and over, not even considering that the heat treating process could be at fault. By the way, if a steel is truly horrible and can't be used or sold, it will not be manufactured, as knives are only a small part of steel use and applications.
The forum posters all chimed in, with mostly incorrect ideas, but more than one mentioned "the straightening process used" by the particular heat treater, a large outside contractor that is mentioned a lot by novice knifemakers. The actual crack was a great indicator of the culprit, as it is big and round, like a dent a hammer would make in wood if you slipped off the nail, after being heated with a torch.
Hammer? Torch? Steel? Heat treat? Straighten? NOOOO...
If your head is spinning at this idea, good for you. A hammer has no place in a heat treating facility; there should be armed guards at the door handcuffing and perp-walking anyone that comes near a heat treating area with a hammer! What about a torch? No torch of any type has any place in a heat treating facility, under any circumstances! What about straightening? Another horrible word that has no place near any heat treater, and no consideration in the heat treating conversation! This is clearly why knifemakers should heat treat their own blades.
I've read about this before; this particular heat treating contractor taking a hammer and/or an oxyacetylene torch to knife blades after and during heat treatment (usually during or after quenching) to "straighten" a blade. This is a horrid error, a sign of total incompetence, and hobbyist, part-time, or unskilled full time knifemakers seem to think this is okay! They mention "localized heating" and this is a despicable, terrible, and destructive technique to attempt to fix a screw up!
Let's get clear and gritty, and I'll speculate about what I think is going on. Understand that this is my opinion based on 40 years of heat treating and knifemaking. You can take it or leave it, control your own heat treating (or make sure your knifemaker knows what he is doing) or accept that someone is going to take your knife blade, when it is most vulnerable and during critical phase transformation, to a dark room, and abuse it with a 5000 degree flame and a ball peen hammerhead on a stick.
The first suspicion is the word "straightening" NO knife blade should ever need straightening of any kind, unless it's hand-forged and only then before stress-relieving and while it's still red and plastic in the forge. If a blade comes out of the heat treating oven or quenching medium with a curve, warp, wave, or distortion, something was done wrong: obviously, terribly wrong. In the thousands of blades I've made in four decades, only a few have been distorted in any way, and I can immediately identify the source and cause. It was always an error, and never the fault of the steel type, the quench medium, or the ovens and furnaces.
For instance, if a blade is not held vertically in the heat treating oven, it can relax, curve, and bend, and when quenched, the curve stays. Another error would be quenching in liquid (water, brine, or oil depending on the steel type) and improper entrance into the liquid or improper agitation. A severe and rare occurrence would be waviness due to an extremely thin grind, an overly thick spine, and the difference in quenching rates. All of these errors are caused by heat treating or geometry faults, and no "straightening" is ever done! The way these errors are corrected is this:
Notice that the word "hammer" and "torch" does not appear in any of the two correction techniques. Even the blade that is fully annealed can be easily straightened and flattened by the pressure of the bare hands, since it's fully annealed and soft. It can be tested against a precision granite machinist's block for confirmation of flatness, re-ground to make it absolutely true, and the heat treating can be repeated.
If a knife blade is in transformation (in ANY part of the heat treating process), or has been hit by a hammer, heated by a torch, or deformed any way during or after heat treating, here's what will happen:
I've seen this before with this same heat treating contractor. What was surprising was to read that the same heat treating contractor had had the same "results" with other knifemakers, yet these guys keep sending them work! Obviously, the heat treating company either doesn't know what their employees are doing, or is producing bad results on purpose! More than likely, individual custom knifemakers are way down on their list of priority process, since they are a large industrial firm with much more substantial clients. Why would any knifemaker keep sending this company blades to heat treat? Why?
Now, here's the real shocker. This particular heat treating contractor is a big name in industrial heat treating. The company has an annual revenue of about $5 million and employs about 45 people. They have been in business since 1979, yet claim over 300 years of experience (this is a fairly commonly bloated claim by companies with more than one person). On their bulleted list on their website, one of their "Why Choose Us" points is that they have "Straightening Capabilities." I assume each employee has their own personalized company hammer...
This is another reason that knifemakers should absolutely heat treat their own knives. Even if the maker can only afford the heat treating furnace and none of the cryogenic equipment, and can only afford to do a conventional heat treat, his results will be known, understood, and completely in his own hands. Even a conventionally treated knife blade is better than one that has been hammered, abused, heated in spots with a torch, and will fracture or prematurely wear, bend, or fail.
One more thing: what if, just what if the knife blade fails due to this "localized heating and straightening?" What if it fractures during use, and the blade injures someone or pops into their eye? Who will be liable for this poorly made and treated blade failure? Will it be the knifemaker or the large heat treating contractor? In these litigious times, this is an extremely important consideration. In all tools, knowledge of correct manufacture and construction operations and techniques bears heavily in a court of law.
As a knifemaker, are you prepared to explain why you trust an outside heat treating contractor after reading this section? As a knife owner, are you ready to accept that your knife was in the hands of an outside heat treating contractor?
I really wish I didn't have to do this; I wish that people representing companies who call themselves professional were just that, professionals. I consider a professional a person who has integrity, who doesn't make flippant, offhand, false, or inflated claims. Unfortunately, in much of knifemaking, there is a distinct lack of professional integrity, and an abundance of people who really don't understand the basis for their profession, and this includes heat treating contractors.
As I’ve stated before, if you are a knife buyer, a person who takes your hard-earned money and expects to purchase a premium knife, you deserve to know every bit about how your knife blade was treated, what to expect in performance, the reasons for the treatment, and why you would spend considerably more than you would for a factory knife. Since this is the case, if you are a knifemaker using an outside heat treating contractor, you also deserve to know every bit about how your knife blade was treated, what to expect in performance, the reasons for the treatment, and why you would spend considerably more for custom heat treating.
You would think that heat treating contractors would be specialists in their tradecraft, that they would know their process inside and out, and that a knifemaker who uses them could rely upon the integrity, knowledge, and deep understanding of the process to produce a premium knife blade treatment. You would think that, but in most cases, you would be wrong.
I watched a group of videos produced by a small knife company featuring one of the most predominant heat treating contractors that knifemakers use. The person who was presenting themselves as a professional began to explain heat treating. While I will acknowledge that most of the basic information he gave was correct, he made a lot of statements that were simply untrue, or were based on misunderstood, misapplied, or faulty concepts or information.
Please remember that I’m not writing about a hobbyist, a casual knifemaker, a beginner, or a novice. I’m writing about the statements made by a professional heat treating contractor, advertising in knife media, online, in knifemaking supply company catalogs, on websites, and praised by knifemakers. This would be someone who you would think you could depend on for knowledge and integrity.
If you think that all professional heat treating contractors know their stuff, allow me to enlighten you with some actual statements presented by one of them on a YouTube video, compared to the truth. Remember, there are knifemakers sending steel to this guy's firm to have it "professionally" heat treated. Sad, truly sad.
You might think that in writing this section, I’m nitpicking terms, concepts, and claims, but I’m simply demonstrating how rife our tradecraft is with people who don’t really understand the metallurgy, yet claim they are worth the investment of your money. Please think hard about the logic, information, and experience of every so-called professional. There are a lot of these guys out there doing a passable job, there are others who may do an excellent job, and there are others who are no good at all. Now you have some logic as to why this is so.


A typical processing of a high alloy martensitic, hypoeutectoid stainless tool steel takes 15 actual steps, and 95 hours.
Note! My new extended T3 process takes 30-33 steps and 172 hours!
Look around on the internet and you'll see plenty of recipes for heat treating and processing of knife blades steels. You can find them on forums, bulletin boards, hobby sites, and knifemakers' own websites, but you won't find any here. The reason that I don't include recipes is that they can only be generalized, and heat treating and processing, including hardening, tempering, annealing, or spheroidizing is vastly different with each steel type, and with each circumstance.
What are these differences?
What to do? This is not so simple, but it is obvious. While I can't speak for other makers, in my studio and shop, I have an established written log of heat treating to keep accurate records of each step of the process. I've developed specific methodologies based on my particular environment, equipment, materials, and knife geometries. It's logical to have and employ some basic testing equipment that any machine shop would have, and to keep records of evaluations along with the recommended process sheets for each type of steel used. It's critical to have knives tested (to the point of failure if necessary) to understand the results of process adjustments. The experience in these evaluations that can only take place by making many knives with a wide variety of materials. These knives should be field-tested and use-tested in the hands of professionals, and that should be reflected in the type of knife, the wide assortment of knives, and the client basis that uses them. There is no better testing than an satisfied client, and decades of satisfied clients is a sobering goal.
For those of you who are interested in just what steps are done, and what time this all takes, please know that my clients deserve to know exactly what has been done to properly process their knife blades, and they are welcome to the details. For the general public and other knifemakers, just to be clear, a typical processing of a small batch (4-8 knives) of high alloy, martensitic hypereutectoid stainless tool steel knife blades consists of 15 steps, and takes about 95 hours. I also have an extended, detailed and developmental process called "T3" which shows some surprising and beneficial results. It takes 30-33 steps and 172 hours, about a week!
Other knifemakers and all manufacturers and boutique shops, as well as all heat treating firms and individuals are not willing to pursue the excellence of knife blade performance this far, but I'm committed to the very, very best performance out of my high alloy knife steel blades. I also understand that most makers only use one or two steel types, but it makes sense to have intimate knowledge of how those steels respond in every way including long-term exposure to the elements, usage, sharpening, and finish potential, something few makers but all clients consider. Above all, it's important to be able to explain to knife clients what these processes are, what they do, and how they effect the blades that the client desires, because the knife client, owner, and user are really the important factor in this discussion.


If you've seen a lot of my knives (I know you have; I can read my statistics), you have seen my declaration of my heat treating method. In my early knives, I simply used the phrase "hardened and tempered," which is, essentially, what heat treating is. As a professional knifemaker, I simply harden and temper my blades, just like everyone else.
—Except my blades and treatment are NOT like everyone else's; not by a long shot. As I developed and improved my process, I went from conventional heat treatment to freezing treatments, to extended freezing treatments, to quenching nitrogen furnaces, to sub-zero treatments, and ultimately to shallow and then deep cryogenic treatments. You probably realize that from the basic descriptions on this page.
This is how it should be; a knifemaker's techniques should continually improve based on his knowledge, equipment, materials, and practice during his career. As I improved my understanding of these high alloy steels, my treatment protocols and steps have changed. I've mentioned before that I don't give out recipes for blade steel heat treatment, not because I don't want to share this information, after all, you are on the largest, most comprehensive website of any singular knifemaker in the world. I don't give out details because most people wouldn't understand them; it's a science that exists with differing results in different steels, different shapes, sizes, lengths, and thicknesses of the particular blade! This is why heat treating en masse isn't a good idea; sending your knives off to a faceless heat treater to be give the "58 Rockwell Treatment" won't enhance them or offer an individualized level of improved performance in the process. And isn't that what custom knifemaking is about?
Other knifemakers will argue this all day, to no avail, since most don't have the processing equipment or practice that gives them insight to these differences. I've got a special project in the works on that; keep an eye out, as this will interest anyone who is reading this right now. Without revealing the details, I will note some simple designation practices I use in my own treatment protocols.
"T3" is the designation I give to Tertiary carbide formation technique; the "T" is for "tertiary." This is a third level of eta carbide development that happens in deep cryogenic environments, due first to primary martensitic development, but followed by days of spinodal decomposition. In simpler terms, "tertiary" designates at least 70 hours of deep cryogenic soaking. You might wonder if and how I've seen primary and secondary carbide development, and I have clearly seen and experienced this, settling on the tertiary level as being the most reasonable and productive at this point in my work. In upcoming texts, I'll detail how all this has happened, and what to see and expect in other heat treating processes, but that's beyond the scope of this webpage.
The numeric designation, typically "3" or "4" is much simpler. It refers to how many individual tempering cycles were performed on the knife blade. I've written before how critically important these cycles are to the development of carbides in the steel, and how multiple temper cycles will enhance toughness, and in some cases the hardness of the steel. Imagine how exited I was to see dramatic improvements in overall wear resistance, making the steel seem like a wholly different material. Truly, it takes on another allotropic form when proper temper cycles are used, and with all of the steels I use, this absolutely requires multiple temper cycles. I will go into more detail about this in my upcoming work, since tempering is not what I thought it first was, back in 1979, when I made my first knife.
For my clients and other readers and researchers who wonder about the designation, I can sum it this simply: Tertiary carbide development in deep cryogenics, followed by multiple tempering cycles designated by a number. Thus: T3 means tertiary carbide formation in deep cryogenics followed by three tempering cycles.
The reality of how these cycles are employed, and what happens between these steps is a book on its own! And, no, I don't plunge the blades into liquid nitrogen for a couple days like an oaf!
Decarburization is the loss of carbon from the surface of an iron-based alloy (in our case, steel) as the result of heating in a medium that reacts with carbon.
The number one error in heat treating is decarburization. Carbon can move in solid steel, and when the steel is heated, it tends to migrate to the surface and react with oxygen, producing a scale of black, burned material. This scale is mostly the carbon that was in the steel knife blade, now uselessly sitting on the surface.
When novice or unknowledgeable knifemakers discuss this scale, they often call it "decarb" and tell others that it simply has to be removed, as if it is some normal, expected occurrence. Know that all decarburization scale represents a failure in the process, and no scale or decarburization should be present on any steel knife blade after heat treating. Knife blades should come out of the process only slightly discolored, with no visible scale, and a simple light sanding with fine grit will be all that is required to remove the discoloration. In the properly heat treated blade, usually a fraction of a thousandth of an inch will have to be removed to bring the blade to bright, full polish.
If there is "decarb," the carbon content in the steel has been compromised. There isn't much carbon in steel to begin with; in most knife steels the amount is from 0.6% to 1.6%. The loss of carbon is highly detrimental to steels, and steel knife blades that have decarburization are, in reality, failures of process. You won't see the word "failure" used by knifemakers when discussing decarburization, but it represents a final, lower content of the most beneficial element in steel (next to iron) and is truly a breakdown in heat treating.
You can't tell if your steel has lower carbon from appearance, particular if the blade is sanded, ground, or finished. The knife blade will still cut, it may still seem hard, wear resistant, and tough. What you will have is a lesser performer, overall. This goes on a lot in knifemaking; blades seem good enough for use, but are inferior to a properly treated blade. When you finally do receive a properly treated blade the difference can be astounding. To know how this happens frequently in our tradecraft, read this section of bad heat treating process on my 440C page.
Decarburization happens for several reasons, and it's entirely preventable, so there is really no reason to tolerate this huge error in heat treating practice of knife blades. The first cause is the atmosphere around the blade during critical heating. Heat treating furnaces can be outfitted with nitrogen or argon inert gas to purge out the harmful oxygen surrounding the blade. Another, more common solution is to wrap the blade with a thin envelope of stainless steel heat treating foil, commonly used in all machine shops. This forms an exclusionary environment and atmosphere around the blade, one lacking oxygen or other harmful gasses, and prevents all decarburization. Combine the two (purge gas and stainless foil) and the blade will come out of heat treating with just a slight discoloration and absolutely no black scale at all.
For steels that must be immediately quenched, like O1 oil-hardening steel, the envelope would slow the process of quenching, so it's not used. O1 is heated quickly to its hardening temperature, in a reduced oxygen or inert gas furnace, and held only long enough for complete transformation, and no longer. When I treat O1, the only discoloration is a medium gray darkening of the surface from the oil it's quenched in.
This leads us to the second cause of decarburization and that is extended time at transformational temperature. Steels that are held for far too long at critical temperature will have excessive carbon migration, and thus, heavy decarburization. When manufacturers suggest holding times at austenitizing temperatures, these are suggestions based on standard steel sizes. While you'll have to contact the supplier's data source for clarification on this, many foundries time at critical temperatures are based on 1" thick blocks of their steel! A knife blade is incredibly thin in comparison, so does not need to be at this temperature for as long as a 1" thick block. Experimentation and fine tuning by each knifemaker will define these critical times. What is important to know is that the data sheets are suggestions, and each machining shop or tooling maker is expected to develop his own process.
For the knife client, owner, and user, know that no decarburization should happen, ever, on your blade. The steel should come out of the process clean, and only slightly discolored (from heat only). How to tell? If your knife blade has unfinished areas that are blackened, there has been decarburization. If the knife is completely finished on all surfaces, you may not be able to tell, so ask your knifemaker about decarb. If he claims it's normal, find another knifemaker, or at least one with more skill.
To illustrate how slight discoloration is the only effect from heat treating, consider this—when I heat treat a fully engraved knife blade, in order to preserve the engraving (done before heat treatment) I can only remove about half a thousandth of material after heat treating to bring the blade to full polish. I grind the blade to 1200 grit before heat treating, then I engrave by hand, and after hardening and tempering, I make a light pass with 2000 grit abrasive to remove the color, and polish. Good heat treating practice will preserve fine engraving cuts, because it takes almost no finishing to bring the knife to a high polish. That's good heat treating!
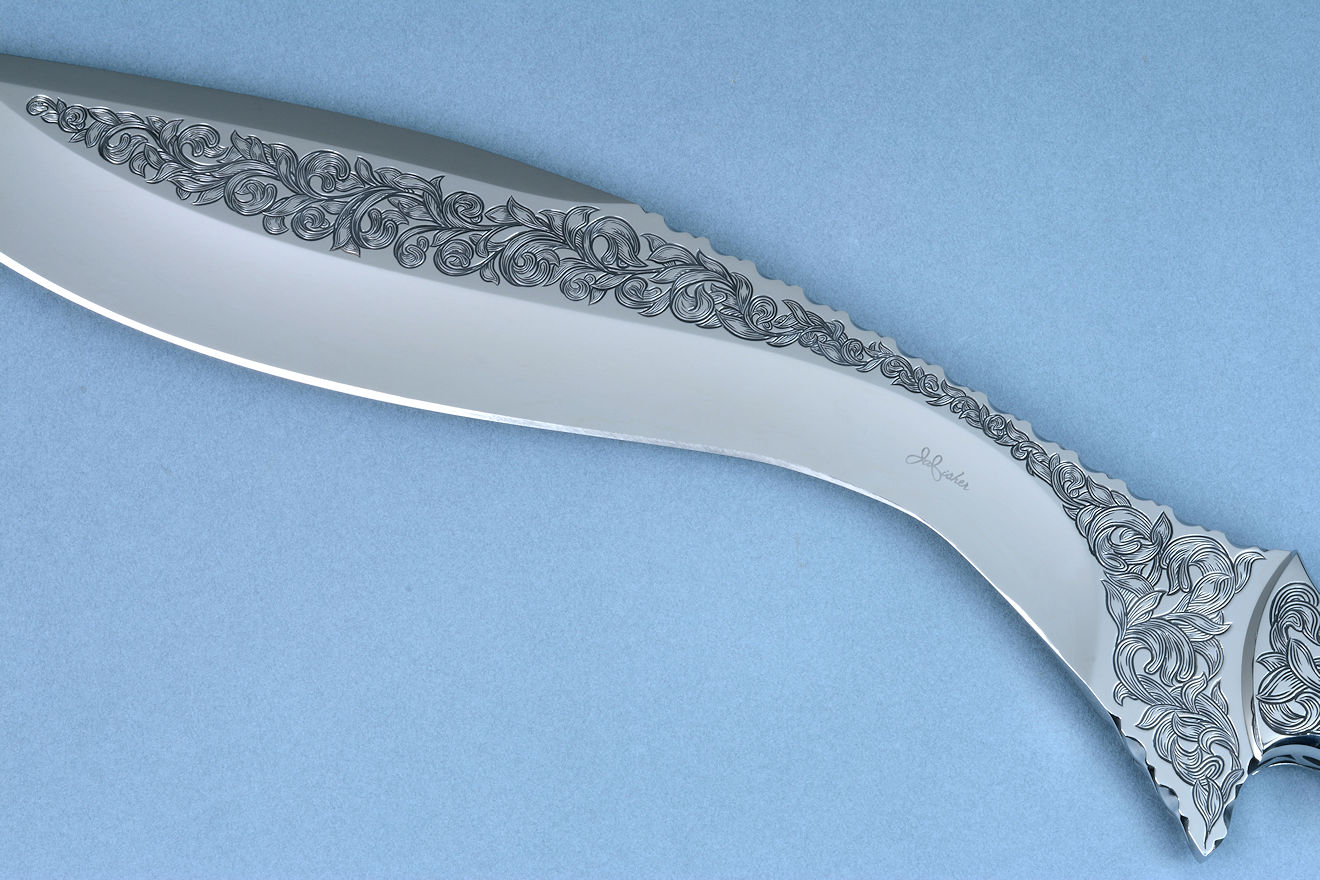

Fine tuning of the process is expected in the professional shop.
You may have read on this page, my Blades page, and on my FAQ page that there are no secret treatment procedures, regimes, and methods that are unheard of in knife blade treatment and heat treating of specialized tool steels. In those sections, I suggest that the maker should follow each manufacturer's suggestions; that it's easier than baking a cake, in that the knifemaker does not have to mix the ingredients, he simply has to process them. For most knifemakers, and for most manufacturers and companies that heat treat knife blades, it's simply a matter of following the manufacturer's directions, because each foundry has spelled out their specific treatment procedure. In the section above, I explain why recipes should vary a bit depending on the manufacturer, date of steel creation, and expectations of the use and application of the steel.
You are on an advanced page now, so I'll take this further. While it's fine to start with the foundry's recommendations, what if fine tuning of the process is necessary or desirable? Like every professional machine shop, the knifemaker is expected to develop his own treatment regimes, to tune the process a bit, to make adjustments that can yield an improvement on the standard foundry or steel supplier's recommended process.
I'm writing about tuning and adjusting the process, equipment, and steps: tweaking them for the best possible results. The maker should start with the manufacturer's process, and then, if he desires, and if he as the time, interest, and ability, he can make adjustments that can yield a higher performance blade, or a more dimensionally stable blade, or a tougher blade than standard process.
For my own work, I have no issue whatever discussing the fine tuning of the process of heat treating and cryogenic processing of knife blade steels with my clients, and have found that they are quite knowledgeable about the subject. I don't offer this information to the public, here on the website, or to other makers, but I do discuss these particulars with engineers and metallurgists at my suppliers and at the foundries I deal with. It isn't reasonable to offer these details to knifemakers, since all of them have different equipment and work environments than I do. Many of them have mistaken and incorrect process steps. For example, a good deal of these knifemakers don't adhere to the cold temperature drop rate of 4-5 degrees Fahrenheit a minute and they just thrust the blade into dry ice slurry or liquid nitrogen, which is ruinous to the steel. A lot of them don't even reach a martensite finish temperature which leaves the steel with metastable (unstable) retained austenite! Nearly all of them short the cryogenic soaking and aging equilibrium step to a handful of hours maximum, even though it's been proven that this is not enough. Because of these mistaken concepts and lack of study and experience on the subject, it doesn't make a lot of sense to share minute details of process with other knifemakers when they don't even understand the basic principles.
After having made knives for over 40 years, my clients expect that I apply certain adjustments to my process overall, and these can be significant and important improvements based on my particular equipment, layout, steps, and heat treating environment. Of course, all changes and adjustments need to be carefully tested and plotted, recorded and evaluated in the heat treating log that records everything done to every knife blade.
What are some of these adjustments I'm writing about? They start with simple things, really. The tuning of a heat treating oven and calibration of the thermocouples compared to a standardized test instrument is and important one. A minute or two adjustment between various blade thicknesses in a batch that contains both thick and thin cross-section steel blades offers improvement. The block quenching apparatus, temperature, and time compared to rate of cooling fluid flow and circulation can make a difference in the steel quality. Peak drop-off times of temperatures between steps and even how many seconds it takes to move the knife to the next phase of the process is important. Cryogenic soak times are substantial players in the process; there is a substantial difference in soak times of 2 hours, 10 hours, 30 hours, and 60 hours. The temperatures and rates of cooling and heating in the multiple tempering cycles are tremendously important, as is the very specific temperature control of the tempering oven and cooling rate buffers. No single component is more important than another; all are critical steps. The entire process is a synergistic reaction of allotrope conversion and control, leading to a superior result.
As with all of these adjustments and tweaks, the client deserves to know the process, if he is interested and inquires about his knife. The knifemaker's recorded log should track the steps and results; fine tuning of the process is expected by the professional. It is a controlled, sophisticated methodology based in experience, testing, logic, and comparison. It can only happen through decades of practice by the professional knifemaker treating his own works, wearing the attitude of the creative logical experimenter balanced with the control of the technician and scientist.


In my own studio, the equipment is built around the process, and the process is built around the desired result.
This section is directed more at knifemakers who may wonder about the equipment used in proper processing of these modern, high alloy, and stainless steels. Knife clients and users may learn a little about why this process is important and the maker's understanding and control of it is even more important.
The equipment used in proper processing of high alloy and stainless steel alloys varies depending on the size, amount, and budget of the individual shop, studio, or maker. I won't go into manufacturing or mass-processing, because you can find all you want about these large pieces of automated equipment on the internet. Manufacturing interests are well-covered in the large tool processing field, and examples and suppliers of this equipment are abundant. It's enough to know that a decent small cryogenic processor can be acquired, brand new, for about $10,000, with some smaller models available and plenty of larger ones to be had for the price of a new vehicle. That's just the cryo processor, not the furnace, quenching stage mediums, or tempering ovens and chillers.
This doesn't work for most small shops, as knifemakers and artists are typically processing dozens of blades a year, and not hundreds. Small volume is the problem, and this is why it's usually uneconomical for the individual maker to outfit his own shop. Small volume equipment is simply not made, not offered for sale, and not available, which is sad, because if someone was making these smaller units, they would probably do well! For instance, furnaces are now available for the small shop and studio, but when I started making knives, they weren't available. Most of us who wanted to heat with electric furnaces were adapting and modifying casting burnout ovens and pottery kilns to do the job. Then, several companies started making knifemaking heat treating furnaces and they are doing quite well today fulfilling this need in our trade.
This means that currently, for the modern small shop, the maker must make first the equipment to do the job. This means custom adaptation of existing or available equipment, equipment borrowed from other industries and professions, equipment not designed for, but adapted to use in the small metals lab, studio, and shop.
There are those who claim that without a "proper" cryogenic processor, the results achieved cannot be as good as shipping the knife blade off to a heat treater who is outfitted with the dedicated equipment. This is not true; if the process is followed accurately and specifically, it doesn't matter what brand of equipment is used to do it; it's about the accuracy and control of the process. Just as it doesn't matter which brand of furnace is used to heat the steel to it austenitizing temperature, just as it doesn't matter what brand of oven is used for tempering: what matters is the rate, temperature, and time.
I will suggest that a reasonable attempt should be made to avoid having an outside contractor heat treat and cryogenically treat knife blades because of the reasons detailed above and throughout this page:
That last one is a real kicker. Say you are confident that your outside contractor has the equipment, know-how, and reputation of reliable heat treating. You are dependent on his name, his work, his method. Fine, but what about if you have a client who asks you to make a knife that is non-standard? What about a client who wants a knife made of a steel that has a wide range of heat treating options and methods (like D2, ATS-34, or CPM154CM, CTS®XHP, or CPMS30V)? These, and many other high alloy and stainless steels have a variety of treatment methods, all to create different results. You should be able to explain these to your knife client, as someone who orders or purchases a fine custom handmade knife expects more knowledge, more information, more savvy from their maker, as these knives are not cheap! As a knifemaker, at the very least, the tuning and tweaking of the process to achieve a fine, specific result is an ongoing learning affair, and it allows a vast improvement over typical "safe" methods of processing. I use the word safe because it's safe for the heat treating contractor to perform a less aggressive heat treat regime to avoid damaging a knifemaker's blade. This, I will claim flatly reduces the performance of the knife blade and it's clearly described above.
Batch processing and cost factors: for the professional heat treating contractor, consider this: a modest and small cryogenic processor can eat up 5 liters of liquid nitrogen per hour. This may not seem like much, but think about this a moment. Consider that it will require at least 30 hours or more of soak time, and a ten hour cool down at recommended rates to get to this temperature. This means that 40 hours at 5 liters per hour and the processor is chewing up 200 liters of liquid nitrogen. There are about 1.8 pounds to a liter of liquid nitrogen; this translates to 360 pounds of liquid nitrogen. In our location liquid nitrogen is currently costing about $2.00 per pound. So this means that using this particular processor rate, it will cost $720.00 to do one run. A conservative heat treating contractor may charge between $10.00 and $20.00 per blade, so in order to just break even with the nitrogen cost, the heat treating contractor must batch process at least 70 blades. But this is not the whole cost; he must pay for the equipment, the electricity, the facility, the transportation and overhead of the business and his own labor. I'm using the keystone rule to suggest that he needs to do 200 blades at once, just to break even and make a payment on his equipment.
If your blade is in the 200 in the batch, do you think that he will do it when it's optimum in timing and without process delays? This doesn't make sense. This is why outside contractors handle huge lots of knives, many from knife manufacturers, and they include some handmade works in the batch. Not a lot of personal attention to your knife; this is expected, after all, it's not the maker carefully controlling the process of each knife blade heat treatment.
Look, I'm not here to slam heat treating contractors, they perform a critical service to the industry. I'm just showing how this works, so that you know what to and what not to expect in this process. If a ten thousand dollar processor and hundreds of dollars of liquid nitrogen per heat treat is not in your budget, there are other means and reasons to build your own equipment for this procedure.
Most makers are familiar with heat treating, and my own decades of experience has proven that simple tools can do the job quite well. The issue is understanding the process. For instance, in cryogenic processing, it's not important what container is used to hold the knives submerged in or suspended above the liquid nitrogen for dozens of hours during aging, what is important is the rate at which the steel reaches that low temperature. Simply dropping a blade into -320°F liquid from room temperature is sure to be a disaster. You don't need a sophisticated cryo processor to slowly lower the temperature; there are other methods, including building quench staging, buffer chambers, and even building your own small batch knife-sized processor if you are inclined! The knifemaker often has to build his own equipment and methods, and as long as the focus is the process itself, he can control his results to a fine degree. In my own studio, the equipment is built around the process, and the process is built around the desired result.
I won't go into great detail about the technical side of the equipment here; that's a discussion for my advanced book, and every artist and craftsman has his own idea about how he would like to proceed, based on the knives he's building. I will acknowledge that a good background and understanding of physics, electronics, and machinery is essential if you are to take this on. You won't find this information on a knife forum; it's too far-reaching and complicated. As with most things, a little knowledge is a dangerous thing; immersion in deep background study must take place first. I will state that if you have read the entire page up to this point, you already have an understanding of heat treating and cryogenic processing greater than most knifemakers or knife manufacturers!
In my studio, the equipment is accurate, verified, dedicated, and tested, and much of it is either handmade, or adapted from other industries for the specific purpose of making knives. It's also like the rest of my knifemaking: continually evolving, improving, adapting, and changing to suit continual advances in steels, in knives, in materials, and in the requests of my clients, who want the very best knives possible from me. This is a tradecraft and art where the learning never stops, and I'm proud and happy to be a part.

"Cryogenic treatment is not proven or accepted method."
It's sad when I read comments with this bent. It shows that the person who made them did not educate himself on the widespread use and benefit of the process, borne out in countless studies, articles, and intensive research. The person who claims this sticks stubbornly to his claim, and no one is going to convince him otherwise, no matter what the facts are, and what reality is proven to be.
"The consequences of a claim that something is true are entirely irrelevant to the issue of whether the claim is true.
--Steven Goldberg
This is how claims and misinformation is spread around the internet and in conversations. Claims have consequences, and those consequences are shared and reinforced by others who repeat the claim, but, after all, it's just a claim.
The reality is much different, and in making fine knives, the reality of results can be physically distinct and inevitably proven, particularly by professional knife users. It's going to be harder and harder to substantiate false and outdated claims in the modern information age, and I'm glad to be living in a time when knowledge is so abundantly available at our fingertips! You just have to be able to distinguish who is presenting facts supported by hard data and research, and who is making excuses and uneducated claims.
Thanks for reading this, and thanks for stopping wives' tales, misinformation, and lies in our tradecraft, interest, and art.


As you might guess, I get plenty of emails questioning me about heat treating and cryogenic processing of knife blade steels, since is something I professionally do. Please remember, I don't perform this service for others; I'm not pushing a business in heat treating; I have nothing to gain in the heat treatment of other people's knife blades. The reason I present this information here is like the rest of this website: I do this as a service to my community, to give realistic, valid information to the reader. Whether the reader is a past, present, or future client of mine, whether the reader is another knifemaker, beginner, hobbyist, part time, or full time, whether the reader is a person who has a passing interest in heat treating and blades or he is a dedicated knife enthusiast who wants to know the facts about the subject, the information I present here is a gift to my profession. Anyone interested in knives in service in real world use, knives made in the best possible way our modern technology can dictate deserves to know what is on this page. While the reader can find this information in professional sources, text, documentation, studies, and guides, I've compiled it here with real world knife blade creation, and include misperceptions, erroneous processes, and mistaken ideas, so that you can know the facts and the hyperbole together. Then, the reader can make his own conclusions and decisions based on this information.
As the traffic has grown on this page and many others related to specific blade steels and knife related process, more questions, curiosities, and interest has grown, and that's a good thing. I've decided that, rather than answer some of these questions and inquires on a singular level, I will post pertinent queries here, if they have any bearing on the topic, for others to learn by. I detail this on my page "Learning About Knives." Please know that I don't ordinarily answer questions not related to direct knife orders; I simply don't have time to answer them all. So if you write me and ask a technical question, please don't be surprised if I don't answer you. Sometimes, a query will strike me as important, in that I can offer what I have learned and know and many others who are reading the same pages will find the answers they are looking for as well. Again, I do this as a service to my profession, and you can learn about the service aspects of the professional knifemaker at this link.
Here are some important emails with the answers I've given. Note that names are omitted to protect the privacy of the individuals, and other knifemakers, companies, and entities are also not given, mostly to protect them from embarrassment. I'll add to this section as I feel is necessary; thanks for helping to stop misinformation, wives' tales, and misconceptions in our tradecraft through learning and education.
Hi Jay.
I have been reading your website regarding heat treating and cryo treatment.
AWSOME write-ups. I’m not finished reading it yet.
I see you are now using some 154CM steel.
Make knives part time, and have been using D2 tool steel. I am still persistent on
having my blades tempered on the
primary hardness point of 450’ish F rather than 925is F. Recently I have read that N***** C********* has been playing
around with various heat treating technics for D2 and CPM3V. He has been deepfreezing his blades prior to any tempering at all.
Have you tried experimenting with heat treatment like this and having the same positive outcomes?
I ask you because you seem to have the proper studio and equipment to alter your heat treating methods at will.
Best regards
G. N.
My Response:
Hi, G. Thanks for your nice words about my site and articles about Heat Treating and Cryogenic Processing of Knife Blade Steels and my page about D2. I actually use over a dozen different steels currently.
A lot of guys try a lot of things, but the very best way to treat high alloy hypereutectoid tool steels is to first follow the manufacturer’s recommendations, followed by a lot of experience (maybe decades) of heat treating. Here are some things that are important about D2, particularly related to the forum you linked: Acetone and alcohol is shallow cryogenics, and barely that, particularly since long soaks are not possible or done with this method. While cryogenic treatment at -100°F will improve the steel’s as-quenched state, it is not the premium treatment for this steel. Deep cryo is, at -325°F followed by a long, very long soak at this temperature. Try 35-50 hours minimum! This long time soak is critical in carbide precipitation, and carbide grains are not large in D2 with this method, but extremely small and more profuse. By the way, the martensite finish temperature of D2 is -150°F, so the -100°F temperature will not not even achieve complete transformation and there will be significant retained austenite! What proper, regulated deep cryogenic produces in D2 is astounding, with an increase of over 800 percent in wear resistance and 25% increase in toughness over conventional treatment! Notably, this is a 400% increase in wear resistance over shallow cryo (or dry ice) and yet forum posters claim this does not happen, which is flatly incorrect, since it's been proven over and again in actual tribological studies by degreed, peer-reviewed professional metallurgists and scientific research. I don't know how a knifemaker can honestly discount what metallurgical scientists have proven, inserting his own conclusions when he has none of the training, equipment, knowledge, funding, or backing of real scientists. This is why it's so important to read, study, and learn from verified sources, not simply post one's ideas and thoughts on a forum comment section. I defer to those sources who actually know metallurgy and have thoughtfully published their results for all of us to read. But you've got to read, research, and study, and a lot of these makers simply don't or won't make that effort.
Tempering is time-process critical, and I won't go into high or low levels of tempering curves, since it depends on the desired allotrope balance. What is missing in these discussions is that for higher performance and the best allotrope conversion, multiple tempers are necessary in the correct stages, with deep cryogenic soaks in between. By the way dipping D2 into any cold solution (water, dry ice and alcohol, or liquid nitrogen) is absolutely the wrong way to handle this or any air-quenched steel! What will happen is micro fractures from shock, invisible to the eye, but contributing to lower wear resistance. All metallurgical references will clearly state that the maximum cooling rate is 4-5 degrees Fahrenheit a minute, not 100 degrees below zero in thirty seconds! I really wish guys who claim to know how to heat treat would at the very least, do some research, plainly presented in industrial sources and by AISI, ASM, ASTM, and SAE….
It’s good you are researching the best methods. D2 is a very finicky steel to process, and most D2 in knife blades is not processed correctly. By the way, D2 should never be hand-forged; if it is, it is ruined steel. Die shops and machine shops professionally dealing in D2 for high performance industrial dies are very specific about their heat treat processes, and most of them will tell you it’s a hard steel to get right. By the way, snap temper is wrong; it’s a crutch for some other failure (too high a cooling rate or timing conveniences leading to fracture-prone blades on a microscopic scale, showing as high wear, being the main reason).
The best of luck in your endeavors; I look forward to seeing your knives one day!
It amazes me how many knifemakers claim to know what can only be seen with an electron microscope or x-ray diffraction (retained austenite, carbide structure, etc.), while not having the access to, training about, or the very equipment used to make these determinations. But, as I've stated before, it's truly sad that our tradecraft is so filled with misperception, ignorance, and myth. Here's some logical, simple and clear help:
If you are a maker and are using D2, start with the manufacturers’ recommendations. They know their steel and will politely tell you how it is best treated. If you wish to improve on their process, research needs to be done in earnest, through AISI, ASM, ASTM, SAE, and other sources detailing real metallurgical studies. There are some great treatises out there about the process, and I encourage you to purchase them, read them, and study them, and they are not available for free, and this is perhaps one of the reasons makers don't study them; they don't see the need to purchase them, but that is where the information resides.
Forums are not typically the place for professional information (sorry, but true), because the depth of data, information, experience, and process applications can not be presented in a couple paragraphs of a post, or multiple posts for that matter. Take this very page for instance; I suppose it will print out to the equivalent of over 100 pages of text, yet barely scratches the surface of steel knife blade treatment information, and each steel is different! This is why textbook-type resources are the best source of information; most of them are over 600 pages long. For some reason, most knifemakers won't bother to purchase these $100-$400 dollar information-rich engineering sources, much less read them several times, frequently access them for the applicable information, and then apply this data to their own work. I frequently use these necessary resources, but could not convey the scope of study on this website, or this page would be many thousands of pages long!
Again, keep it simple. Start with the manufacturer's recommendations, which are usually the best for basic, reliable performance, and if you wish to improve on them, talk to the metallurgists and engineers at those companies, be prepared for the discussion by studying as much as you can; as most are professionals with degrees and are accredited in their field, and won't appreciate wasting time educating you.
Here's a great formula for success:
Study, study, study, practice,
Study, study, study, practice,
Study, study, study, practice,
Study, study, practice, practice,
Study, practice, practice, practice,
Practice, practice, practice, and then write.
Knifemakers who do little study, minimal practice, and then write on forums are shortcutting a few steps...

I do not know if this question was already made but I would like your opinion about this course. Looking a bit at the snowstorms that finally hit the nation, I noticed that the train rails were literally under the snow and my question is this one, that cold for almost four months and, the consequent temperature change every time it is subjected to the passage of a quantity of wheels also of steel. would all this event have an improvement in the hardness, toughness or tenacity of that particular steel?
Ok, it is possible that this question does not have an acceptable scientific level, but it does not give you curiosity given that there is so much literature about the exposure time below zero and cryogenic and the duration and quantity of those stages ?? As I can see here we would be facing a number of stages for 120 days or more ... not to mention Alaska of course.
Juan H.
My Response:
This is good question, considering the long, cold cycles of winters and exposures, since it will allow some perspective of important temperatures and steel types.
The average temperature of the the coldest occupied place on earth (Oymyakon, Russia) is -58°F (-50°C), which is very cold indeed. It's best to consider an average of this temperature, since spikes and valleys in the temperature will translate to an average as steel cools and warms. I don't know if there is a railroad there, but for the sake of our consideration, let's assume there is.
In the 150 years of rail, we have studied and adjusted rail metallurgy to a great understanding. Actually, tool steels have not been made as long as rail steels, and high alloy tool steels including stainless and high speed steels have only been around less than 100 years. The truth is, we know more about rail steels than tool steels!
Railroad rail is pearlitic steel, ferritic steel, or in newer rails, bainitic steel. It is not generally martensitic, so really doesn't apply to the idea of cryogenic aging like tool steels do. The traditional rail steel is a near-eutectoid pearlitic microstructure, made that way on purpose. Rail steels are made and processed to be tough, only moderately wear resistant, and not brittle. Since the carbon in pearlitic steel is tied up in the layers of iron carbide (cementite) it is not going to migrate and change into eta carbides or any other carbides simply by exposure to slightly cold temperatures.
I wrote "slightly cold" because though 50 below is a brutal environment to humans, it doesn't even approach shallow cryogenics which starts at -125°F (-86°C). Even colder is deep cryogenics at -320°F (-195°C), which does not occur naturally anywhere on this planet.
Back to the microstructure. Since pearlitic steels, ferritic steels, and bainitic steels are fixed to the equilibrium (static condition) of their environment, and the carbon is already locked up in its structure, there is little free carbon to move, wander, relocate, or coalesce into another structure. These steels are generally made with a lot of manganese and silicon to strengthen the ferrite, and are low in chromium, vanadium, or other "tool steel" alloys to give them great toughness and strength with limited wear resistance, particularly when compared to tool steels.
Railroad rail wears, continually, and the wear patterns are heavily studied and analyzed. The compression of wear, the "skidding" and frictional components usually produce a hardening and flaking of the surface, and railroad rail must be periodically reground for proper shape. Just do a search for railroad "rail grinders" and look at this lovely equipment. It's neat to see these massive grinding engines and cars, sparks flying, followed by lighter and lighter grinding, with a final water spray car to knock out any fires. Where I live, more than 60 freight trains a day rumble through, and the track is a continual focus of maintenance.
In simple conclusion, long term exposure of railroad track to moderately cold temperatures would not cause much effect; the temperatures are not very cold, the steel is of low alloy, the condition of the rail is stabilized and relatively soft at about 342 Brinell or 37 HRC.

Mr. Fisher, a question regarding multiple tempers, if I could. Is there any indication from a given steel's alloying mix as to the need for a third tempering cycle, or is it solely indicated by empirical testing? Comparing the whitepapers for AISI-D2 and CTS-XHP, for example, Crucible's AISI-D2 datasheet calls for a double temper, while the CTS-XHP datasheet calls for only one. Your logic regarding the need to temper the newly formed martensite (from the previous temper) makes absolute sense and is in line with what I have read, but I am puzzled as to why the whitepapers wouldn't call this out more specifically. Thank you!
Michael D.
My Response:
This is a really great question. Unfortunately the data sheets supplied by nearly every steel supplier are not detailed, not specific, and are only generalized. They may do this for several reasons:
Now, about the specific metals you asked about, (D2 and CTS-XHP). These are both fantastic performers, and I believe that Carpenter does not detail advanced treatment for some or all of the reasons I described above. Carpenter doesn't even use modern terminology, and mentions "cold treatment" as a possible improvement of their steels. The terminology of Conventional, Shallow Cryogenic, and Deep Cryogenic are the terms used by The Journal of Materials Processing Technology, The American Iron and Steel Institute, the International Journal of Emerging Technology and Advanced Engineering, and other various research sources (some detailed below in References).
I hope this company can get on board with advanced treatment protocols, since they really do make (or supply) some great steel!

What if you don't have all the equipment to do this? What if you don't trust outside contractors?
Hi, Jay.
I've studied again your web site discussions on heat treating and cryo (and will be going back again and again...),
and I have to admit I'm frustrated. I want to work with stainless (I have a knife I started with 440C) but there's
no way I can afford any processing equipment at this time. I'm now sufficiently worried about sending it out for
treatment. For one thing, I want to do ALL the work on my knives, including eventual engraving, sheaths, etc., but
now I'm afraid to to send it out for fear of mistreatment.
So, the question I have is this: is it even worth it to work with stainless at this time? Until reading your work, I *was* going to snap temper and treat with dry ice and RV fluid (based on forum posts and youtube videos). Now, I'm seriously wondering what direction I need to go.
Again, I've enjoyed thoroughly your videos and web work. It has caused me to rethink just about everything I do. By the way, I saw that you're working on your book, and I'm looking forward to that. I'm confident in my ability to build what I need (I've built my own heat treat oven, for example).
Thanks for the information you've shared.
-D.
My Response:
This is a great question, and D. has the right frame of mind, being seriously concerned about sending his knife out for heat treatment. The reality is that he won't know exactly how his blade is treated, and thus, can't let his customer know exactly how it was treated and what to expect in performance. If a knife blade fails or doesn't hold an edge to the standard of the knife owner, it will be D. who has to rectify it. The client holds the knifemaker responsible for all parts of the knife, and not some heat treating company.
It's true that heat treating processing equipment is expensive, and there simply is no way around that. Knifemaking has been focused on the mantra "cheap and easy" for far too long, and this has left us with hand-forging 19th century techniques and low alloy carbon steels because all that is really required to make a knife is something to heat up some steel, something to pound it on, and something to pound it with. This is fine for third world countries, but this is not applicable in my world, where the finest steels require advanced treatment protocols.
But here's the thing: if you go to any 440C steel supplier website, they will only give instructions for conventional heat treatment of 440C. They'll give you a hardening process (heat blade up to 1850-1950°F and air quench), and a tempering process (temper at 450°F for a 58 HRC hardness). They won't give you details about quenching in oil intermittently, they won't tell you that you have to quench 440C to cryogenic temperatures for full martensite finish temperature; they won't even mention a long cryogenic soaking for eta carbide development. They never mention multiple tempering cycles, and never suggest cryogenic cycling stages between tempers.
For the steel supplier, conventional heat treatment is simplest, and the simplest process for their clients. As I've said on the site on this page, conventional heat treatment, properly done, will yield a steel knife blade that excels in every performance aspect to a hand-forged, or low alloy, or carbon steel blade. Steel suppliers know that most metals shops don't need the extremely advanced treatment protocols, and it helps them that if the steel is less durable and used up quicker, they'll sell more—simple, isn't it?
This is an issue when a maker wants to move from merely making knives to making high performance works throughout. He won't find the process cheap and easy; he won't find the data easy to come by; he won't find the endless details of procedure and testing for verified results in a simple way. We live in a time of incredible alloys, but we don't have simple, cheap, and easy ways to process them. There is a lot of misinformation (particularly on knife forums and YouTube), and it does our tradecraft no good. At least D. and others are recognizing this.
So, what to do? How does D. make a better knife?
Here's my recommendation: Stick with the 440C, it's a great steel. If all you can afford to do is a conventional heat treatment, then do it. You'll be surprised that even with a conventional, recommended simple heat treatment, 440C is a great performing steel. Do the very best conventional heat treatment you can. That means speedy, quick movement between stages, accurate and specific temperatures and soak times, and clean and intelligent process. Most of the mistakes in processing of 440C are bad ovens, incorrect temperatures, incorrect soaking times, incorrect or bad tempering times, atmospheres, or environments, and singular temper cycles when multiple tempers are needed. Work cleanly, efficiently, and log every step in a record book so that you will be able to repeat the good process, or identify any errors and not repeat them. Think like a lab technician: eliminate variables, and do your best process. Then, test the blade yourself and have your clients test it in use. Tell them exactly how you have heat treated it, and learn from it. They will appreciate your honesty and return as clients.
Next, as your work continues, analyze it and make minor improvements to start tweaking the process and results. KEEP ACCURATE RECORDS. You'll start tweaking your equipment and process, and start that with what you can afford, like an accurate pyrometer set. Don't temper in a heat treating oven; it's too inaccurate! Don't temper in a home oven because it's too inaccurate with wide hysteresis swings. At some point, you might try a heated oil quench to bring the steel faster into martensitic conversion (this can work with thicker 440C). Start long soaks in a sub-zero environment; this does help in the condition of the steel, even though it's not cryogenic. Don't bother with alcohol and dry ice; you can't control the temperature drop, and you can't maintain it for long enough. You might be surprised by what you learn if you quench a 440C blade, and leave it at -20°F in a mechanical freezer for a week before tempering. Use your hardness tester; you must have one to do any heat treating work. Read a lot, but beware of what you read and who is posting it. A guy who sells knives for $250 has a lot lower standard than one that sells them for $4000 to counterterrorism professionals, for instance.
The truth is, D., just by educating yourself and working with a hypereutectoid high alloy steel like 440C, and doing a steel foundry recommended conventional heat treatment, and disclosing this to your clients, you'll be doing better than most knifemakers and knife manufacturers, who don't know or disclose their process, steps, and procedures! The upgrades to equipment, process, and results will come, one knife at a time.
It's been my experience that there aren't enough really good knifemakers around, so become one!
Jay

Good steel comes in all shapes, sizes and applications. A steel that is designed for and performs well in one application does not translate to a worthwhile use in a totally different application. Here's an email that someone sent wondering about making a knife with deck plate steel.
Can you work with STS (special treatment steel) aka high nickel steel to make me a number of knives? I have some discarded sts steel from an Essex class Navy ship and would love to have you create a family heirloom I can pass down to my children. It's extremely heavy and I don't know if the type of steel it is would limit you.
Thanks,
--M.
My Response:
Hi, M. Unfortunately it's easy to get different steel applications confused. When you think of a tough steel that was used on battleships for deck plating, you might think that such a superior steel would make a great knife, simply by its pedigree. Nothing is farther from the truth.
STS steel is the acronym for "Special Treatment Steel," and this is literally protective deck plating. It's designed to absorb the energy of an impact. When you think of the physics of this, consider that a hard material might well shatter, but a softer, more ductile material would deform and in the deformation, absorb the energy of the impact without letting it transfer to the personnel or equipment below. So STS steel is designed, by metallurgy to be very ductile and forgiving. Ductile means the ability to be stretched into a wire, so you can see that ductile is, in layman's terms, soft.
Looking over the actual alloy content gives some great direction here. The most important and first element amount to consider is the carbon. STS steel has 0.35% to 0.4% carbon and this is very low carbon. It's clearly hypoeutectoid, and the exact percentage keys me into the fact that though hardenable, the martensite finish temperature is just about at room temperature, so in hardening operations, simply heating it up and quenching it in water would bring it to its maximum hardness, without sub-zero or cryogenic processing. The small addition of chromium (1.75% to 2.0%) would add a little bit of hardness and durability, and this is good, but the big red flag here is nickel.
When you see notable nickel content in steels (STS has 3% to 3.5%, and that's quite a lot), the first thing that should pop into your head is that nickel is an austenite stabilizer. Nickel is put in steels mainly so that they will have higher levels of austenite after treatment. Austenite is not a strong, hard structure; it's a tough, ductile structure. In knives, machinery, and cutting tools, austenite is what everybody is trying to avoid, since it is detrimental to the steel tool performance. This, of course is only in tool steel application, and not in deck plate application. In a deck plate, you would want the steel to retain high formability, ductility, and have some limited strength, simply so that you can mount things to it (like the rest of the ship and equipment).
Simply put, STS is the wrong steel for any tool application of any kind. No amount of heat treating and knifemaking skill can make it into a worthwhile, useable cutting tool, but I'll bet it does polish up real pretty, with a finish kind of like a less corrosion-resistant version of 304 stainless. For that heirloom, you might polish it up and engrave your service dates or the ship that it came from on it. If you have a lot of it, it would make a great workbench top: strong, tough, and resilient, and better than low carbon steel. Just a thought.
Jay

Here's a great email from a chef who has invested in learning about heat treating in his search for the very best, high performance knives:
Hi Jay,
Just a quick note thanking you for your recent YouTube videos! I've learned so much about heat treating
and knife steels from you. Unfortunately, I think they ruined me. I just got off the phone with another
custom knife maker who uses 440c in most of his knives, and he was bragging about his heat treat. He says
he gets his blades to 63 HRC by quenching them super fast.
In the back of my mind I could hear you cringing and reiterating the 4-5 degree F temperature change per minute rule for optimum Martensite formation. He didn't even mention the Martensite finish temperature or carbide formation at shallow/deep cryogenic temperatures.
Needless to say, I now have a very limited number of knife-makers I can buy from in good conscience. I know you're very busy, and I don't want to sound desperate, but PLEASE, post some chef knives on your website soon! (ok, I admit it, I'm desperate)
Thanks again for your videos and all the great info on your website. I hope to be a future customer soon,
--A.
My Response:
Hi, A. Thanks for writing; I understand your dilemma!
It’s not my intent to spoil knife owners and users to only the very best premium treatments; I know that few makers and no manufacturers perform premium treatments; I just want people to understand the range of processes and why some are better or worse than others. While it would be great if knifemakers thought more like metallurgists and scientists than casual craftspersons, I realize that this is not in the scope of many knifemakers’ interest.
As far as the individual maker you’ve mentioned and his process, I do hope that in the videos I’m being clear, and I’ll touch on this very subject in the next video that I do. Initially, the quench from critical temperature to room (ambient) temperature is fairly fast (and not 4-5 degrees per minute). For 440C, simply leaving sit in still, open room air is typical, but in some circumstances, 440C is actually quenched in oil. The slower rate yields a slightly lower hardness, and the faster rate of an oil quench gives a slightly higher hardness, but comes with the possibility of warpage, quench cracking, microscopic fracture, or internal stresses in the steel that can lead to problems down the road. Typically, the oil quench is reserved for thicker cross-sectional areas. The 4-5 degrees Fahrenheit per minute guide is for the transition from ambient temperature to cryogenic temperature, after the initial quench to ambient. Of course, if he's not going cryogenic, the 4-5 degrees per minute rule won't apply...
Did you know that the critical (austenitizing) temperature has a lot to do with “as quenched” hardness? In any case, the hardness when quenched only to room temperature is not a valid indicator of success in heat treating, since the martensite finish temperature target of 440C is in the cryogenic temperature range. If the maker is simply quenching quickly to room temperature and then moving to the tempering cycle, he may have an adequate blade, but he will have about 29% retained austenite!
This is why conventional treatment is not premium treatment. The steel may test hard, but hardness testing alone does not equate to wear resistance and longevity of the blade. Of course, if there is no cryogenic processing, there will be no benefit from the eta carbides that result from cryogenic equilibrium (long term soaking) and the blade will be markedly less wear resistant overall and not as tough, no matter what the tempering treatment is.
Again, there is no right or wrong way to process, just substantially different methods with substantially different results!
For my own work, please know that I do have many chef’s knives in the works; a couple are completed and waiting their accessories (stands, rolls, sheaths, or cases) and some are waiting on others that will be joining their groups. Some knives are singular, some are in pairs, some in threes, and one group I’m working on has a dozen different style of chef’s knives—I’m working on it!
Thanks, sincerely,
Jay

In a follow-up email, I was queried about quenching 440C in oil. Some white papers and data sheets include this as an option of quenching. The steel suppliers don't often describe why this is listed, so I thought it was important to include it here.
Do you ever quench your 440C in oil, depending on the intended use of the blade? You are truly a blessing and treasure for then knife-making community. Thanks for posting my email to your website too. I'm honored to be part of your discussion to bring knowledge and clarity to knife enthusiasts around the world.
--A.
My Response:
The reason that someone would quench 440C in oil is more to gain a deeper hardness in thicker pieces. In a thick piece of steel (think 1” thick), the rate of cooling at the surface is fast enough, but deep within the steel, the rate is much slower. This is because steel is actually a relatively slow conductor of heat, unlike copper, silver, or aluminum which conduct heat quickly. So the heat isn’t carried away as fast in thicker stock, relying only on conduction. In order for the higher quench rate to occur deeper in thick metals, sometimes the oil quench is used, with the possibility of quench cracking. However, since the stock is thicker overall, quench cracking would be diminished as there is more metal to physically support the steel. The only case where this would be an issue would be in steel parts with complex geometries, and knife blades are not complex geometries.
In 440C knife blades and in my own experience, oil quenching isn’t critically necessary. Knife blades are rather thin, and quench and cool adequately without the added and unnecessary stress of oil quenching. Remember, I'm not writing about oil-quenched steels like O1, but air-quenched, high alloy martensitic hypereutectoid stainless tool steels.
Consider that, in still air or between properly designed liquid-cooled quenching blocks, a 440C knife blade will lose 85% of its latent heat energy in less than 30 seconds, and that's a lot! More important is a smooth transition to colder and colder temperatures, without pause, and into the cryogenic range. Transformation continues until maximum martensite conversion is achieved. Then, held at cryogenic temperatures, eta carbides start to coalesce very slowly at very low temperature equilibrium.
To get a little more technical, once the nose of the TTT curve (isothermal transformation diagram) is passed, there is more time for the steel to be cooled at a slower rate. I don't want to get into this deeply, since this is not my metallurgy class, but generally, the addition of alloying elements increases the hardenability of steels by moving the nose of the isothermal transformation diagram to the right, allowing slower cooling rates for alloy steels to form martensite. This is why these high alloy steels are air-quenched in the first place. The same alloys lower the martensite start and martensite finish temperatures considerably, thus the necessary cryogenic quench.
It's important to consider that fast quenching introduces residual stresses that may not be eliminated by further processing (tempering cycles). Quenching a steel too fast can lead to less-than optimum steel quality and introduce stresses in the microstructure that are undesirable and unnecessary. Some makers use a faster quench as a crutch to introduce higher hardnesses, since they think that the hardness penetration tester is the only indicator of steel quality; it is not.
Simply put,
For my metallurgists: the optimum treatment for any hardenable steel is to quench at the slowest rate possible to pass on the left side of the "nose" of the TTT curve (isothermal transformation diagram), and stay on the left side of the transformation line until complete martensitic conversion. Eta carbide formation under cryogenic compression comes later...
Thanks,
Jay

I should have a section on my website called, "Knifemakers say the dumbest things," to illustrate the many wives' tales and myths and misunderstandings in our tradecraft. As a professional, I would be remiss if I let these foolish fallacies stand, and I feel obligated to detail where and who they come from. There is a bit of truth in all myth, but after you read this section, you'll understand where it comes from and why it's a myth. That will be the limit of what you will learn, for there is no truth to this myth at all, just a lack of understanding and lack of skill in knife blade sharpening, and lack of skill in making knives.
Hi Jay,
Can high alloy stainless steels get as sharp as high carbon steels? I understand they can hold an edge longer, but is that edge as keen (hair popping, shaving sharp)? I've heard some knife-makers and blade enthusiasts say the sharpest steels have the finest grain structure, aka Hitachi White #1 steel. I is there any truth to this?
Thanks again for putting up my questions, your opinion means a lot to me.
--A.
My Response:
Hi, A.
There is nothing wrong with carbon steel; there is nothing wrong with high alloy stainless steels. Both of these types of steels can be and are used to make knives. There is, however, something wrong with claiming that one can be made sharper than another. This is simply untrue.
The "sharpness" comment is a horribly common misconception, and a total misunderstanding and myth in our tradecraft. It's so pervasive that it will take decades to get rid of this foolish idea. It's sad, really; makers are supposed to know and understand steels and cutting edges, and they spread this error continually. It degrades our profession and contributes to the idea that knifemakers are uneducated and unknowledgeable hicks.
In this response, I'm going to detail where it comes from, who is spreading it, and why. I'll also include the truth and some helpful links as well. Though you'll hear this type of comparison for the rest of your days, you won't be suckered into believing it. If you want to skip all of this detail, just ask yourself why scalpels and razor blades are made of stainless steels.
So, what does a professional do about this? For the misconception and persistent myth, you are reading the very solution—education. That's the best I can do for those who will read and think.
But what about sharpening? It's really easy, and you might be surprised. I never, ever, ever use any of the traditional rocks to sharpen a knife and I haven't for many years. I use diamond. It's still a rock, but it's the hardest rock that you can buy to sharpen any knife, and it will absolutely sharpen any piece of cutting metal there is. It's what we machinists use to sharpen our solid tungsten-carbide cutters, and our ceramic metal lathe and insert tools and there is nothing that diamond can't sharpen! More importantly, diamond abrasive technology has progressed so far that many vendors sell diamond sharpening "stones" that are cheaper than silicon carbide or aluminum oxide stones! And they cut 1000 times better! With a diamond hone, you can get a razor-keen edge on any high alloy steel, and because the high alloy steels can be made thinner, are more wear-resistant, and are tougher than carbon steels, you absolutely will have a sharper, better cutting edge for far longer than any carbon steel. And because they are stainless, the cutting edge won't corrode away!
IF YOU DON'T BELIEVE that stainless steels can be made razor-keen, and you shave your hair on any part of your body, consider that the steel used to make that razor blade you use is probably AEB-L (Sandvik 13C26, Jindal JBS), a low to medium-carbon stainless steel. What? Stainless steel is razor-keen? By the way, this steel type was invented in 1920, and hasn't changed in almost 100 years. Something to consider when people disparage new stainless steels or claim that "440C is so 1990s." Consider also that all scalpels used in operating rooms have martensitic chromium stainless steel blades. This wouldn't happen if they couldn't be made incredibly sharp.
Some things to think about: if carbon steels and low alloy steels truly were incapable of achieving and holding a fine cutting edge, why would industries even make high alloy steels? Why wouldn't carbon steels be good enough? Sure, you could sharpen them more often, but you would get a better, sharper edge, right? So what if you have to sharpen a metal shear blade every couple minutes or a planer blade or a wood shaper blade, or a textile cutting blade? Just think of how quickly you could hone up a fine edge on that carbon steel and how impressed you would be. Of course, you'd have to stop the production of the machine every couple minutes to sharpen it, again and again.
Now, about grain. The size of the grain has no bearing on sharpening a knife blade, unless the maker has screwed up royally and allowed tremendous grain growth by missing the heat treating marks (time, temperature, and process) completely. Read more about grain and sharpening on this humorous section on my Blades page.
One more thing: anybody who calls a particular steel alloy or brand by the color of its paper wrapper doesn't understand the wide array of technical steel details and is trying to charm their readers by casual terms. Engineers, scientists, metallurgists, and machinists don't use paper wrapper terms. Even in Japan, they have their own steel designation system (it's called the JIS system) and they don't name steels by their paper wrapper colors either; only knifemakers and Japanese knife companies who want to spread some special mystical mojo do that... I like purple steel; it's the best! White steel must be very pure... blue steel is hotter than an ember, and red steel is like the scorching fumes of Mercury! See how casual words have no business in the steel sciences?
Okay, I hope you have a sense of humor-
Thanks,
Jay

Recently, I had the honor of speaking to a graduate level metallurgy class at a university. They were are great group of minds, and I hope to continue this in the future. One of the participants (Joshua) asked if I followed any standards (like ASTM) for heat treating practice, or if I've honed in on my own special treatment regime in working with high technology knife blade steels.
I thought that the question was important enough to address here, for Joshua and other interested participants and readers for some more clarity. When I answered him, I immediately went into the description of manufacturer's white papers and data sheets on heat treating, and didn't really talk much about ASTM standards. Did you know that there are six different types of standards supplied by ASTM? They are Test Methods, Specifications, Classifications, Practices, Guides, and Terminology. One could write their own book on just how the ASTM works and its critically important place in the safety and use of our modern materials.
There are many standards in our world, because materials need some regularity, so that the uses are predictable and valid. Since we have so many materials in humanity, organizations have been developed to offer a standard or regular way of identifying, regulating, and categorizing how they are worked with, so we know what to expect.
After discussing this with engineers who actually have worked on some of the ASTM's committees and boards, it's clear that the organization offers general guides and specifications, and often refer back to the manufacturers and (in this case) steel foundries and suppliers for the details on how to treat their products. This is because—although the ASTM sets standards—they often encompass a wide range of the material's makeup, percentages, and condition.
For instance, it's standard for 440C to have a range of chromium content, from 16% to 18%, and this is a large difference. Rather than detail each foundry's specific product for a highly specific treatment protocol, the best and most detailed information comes from the particular manufacturer. The manufacturer or foundry offers the starting points for treatments, but even they don't give highly detailed information on steel treatment.
They do this for many reasons, but consider that they don't know exactly what is being made with their steels, so it's expected that each metalworking industry will develop the high details of heat treatment based on the exact use and condition of the steel product being made as well as the expertise of the metals shop, their equipment, budget, and time constraints. I've mentioned these limitations before at this bookmark, but since the foundry or steel distributor doesn't know what happens to his steel, he can only generalize.
Another important point is this type of disclaimer. For educational purposes, this comes from Carpenter Steel® data sheets:
"Disclaimer:
The information and data presented herein are typical or average values and are not a guarantee of maximum or minimum values. Applications specifically suggested for
material described herein are made solely for the purpose of illustration to enable the reader to make his/her own evaluation and are not intended as warranties, either
express or implied, of fitness for these or other purposes. There is no representation that the recipient of this literature will receive updated editions as they become available."
From this, it's clear that these are generalized starting guides and not rigid, specific, and highly detailed or regulated documents. This is why it's so important for knifemakers to do their homework, refine their process, and keep accurate records and documentation of results to improve their heat treating methods.
One more important point: there is no ASTM standard for hand knives, anywhere. I detail this on my "Knife Testing" page at this bookmark.
Thanks again, Joshua, for your great question!

At the same University speaking event that I detailed in the previous topic, Matt, a Teaching Assistant, asked about D2 steel. He revealed that there was a lot of variation among knifemakers in the timing and treatment of D2 and there wasn't any specific and regular method for treatment of this steel.
I did detail some of my processing of D2 steel treatment during the session, but I felt it was important to go a bit deeper here, for Matt and other people interested in this very special tool steel. D2 is a finicky, special steel and it's hard to get right. First, D2 should never be exposed to any oxygen during heat treatment; it will readily and quickly decarburize, ruining the possibility of bringing this steel to its zenith in performance.
Matt (and others who want to know), please look over this great study into D2 and cryogenics, done in 2012. I won't include a direct link, since web URLs change, but put this in your search engine:
"Comparison of Effects of Cryogenic Treatment on Different Types of Steels: A Review"
by P.I. Patil and R. G. Tated.
In this study, they stopped the cryogenic equilibrium at 40 hours, and thought that 36 hours was optimum, but my own research has demonstrated a different and improved character of high alloy hypereutectoid steels by bringing this time up to and past 60 hours. Of course, my setup, equipment, range of austenitizing, and procedures are different than in the study. Also, using only the hardness tester at the end of a cryogenic soaking cycle is not the true indicator of what will happen with the wear resistance, since so many more developments happen in the multiple stages of accurate tempering!
This (and other studies) demonstrate that this is truly cutting edge research, so much so, that I'm developing some detailed and specific equipment just to test what happens to these tremendous alloys at these fascinating ranges of treatment.
Graduate level metallurgists and researchers like Matt stand at the crossroads of high technology knife blades, and have the potential to move our tradecraft, science, and art into new realms, never before conceived in modern knives. As I've written before, there is truly a possibility for a knife blade to last 100 years or more, in use. What a great time to be in this field!

You may be surprised to find that I delete ridiculous comments from my YouTube channel. The reason I do this is that what I present there—just as what I present here—is meant to be educational. When you present data, experience, and information like I do, please understand that I do know quite a bit about what I'm writing, talking about, and presenting. While I can't know everything about, say, every type of steel, I do know about the types of steel that I use in making knives, and am quite well-schooled in the real world of making knife blades. From this very page, you can understand that it is not a simple science, and takes quite a bit of dedication and education to understand the process. Just to give you a clear, small representation, just one of the advanced steel properties textbooks that I reference continually is written by top metallurgists through the ASM and costs more than most other maker's knives!
I don't want to get into deep, detailed arguments on the YouTube channel response string with people who clearly do not have an understanding of the science. Doing so (arguing) will tend to make YouTube just like the forums, where divisive, uneducated, inexperienced, and unknowledgeable forum participants spread misconceptions, misunderstanding, and lies about steel properties. They love to argue, post, and present their opinions without reference, logic, or science, and then hope to have what they deem is a conversation when others respond. This almost always degrades into attacks, much of them personal. It is of zero use to those of us who are trying to actually educate and share their experience.
This is one of the reasons I don't post on forums but rarely, and I absolutely do not recommend them for any viable information. Do not get your information from a forum; the very best makers and researchers do not post there, and you will not find the correct information about steels, heat treatment, and knives there. You will find it in metallurgical science textbooks, graduate level studies, peer-reviewed journals, and serious scientific investigations.
A large part of the issues with the internet are in your ability, as a reader or viewer, to understand and delineate truths from ignorance. There is more ignorance than truth on the internet, so you have to think about what you are reading, who it comes from, what their experience level is, who they make for, and what they present. Always suspect, and check sources! Getting information from anonymous sources or hobbyists is a poor result all around.
Below I'll give some examples of a posting I deleted, and I'll give you some insight—for educational purposes—so that you can understand the ridiculous amount of effort it would take to educate someone who has only casual, peripheral knowledge about steel. You'll understand why I don't attempt the effort, since it would require erasing what the person thinks they know about steel, and then starting over from the beginning, "This is an atom..."
I simply don't have time for this, and neither do you. Please accept my sense of humor at this ridiculousness.
"For most steels you can actually induce precipitation hardening (ie of ultrafine carbides) without tempering by holding the steel at around 275F. I've seen steel manufacturers that recommended holding the steel at that temperature for a week, although that's certainly something you'd want to test. I suspect that a lot of the inconsistent results and anomalous hardening that may occur during tempering (post cryo) is due to that effect."
My response:
No, "most steels" are not capable of precipitation hardening, and it does not produce "ultrafine carbides." This guy doesn't have a clue what type of steel he's writing about, yet goes on to interject what he thinks about what might occur in cryogenic treatments... sigh. It is embarrassingly ignorant; so where to start?
PH steels are not knife blade steels; they are completely different types of steels, for different uses and applications! Precipitation hardening can apply to a lot of metals, including aluminum, and some stainless steels are precipitation hardening steels. PH steels are used for pumps, valves, pipes, and tanks that are expected to have high corrosion resistance and higher strength than austenitic stainless steels (304, 316, etc.) They are corrosion resistant steels (stainless) and are 3-4 times harder than austenitic stainless steels, but this isn't even in the same field as tool steels, much less hypereutectoid high alloy stainless steels. They are designated by the identifier "PH." The only one that is classified as martensitic is 17-4PH, so let's look at the very best precipitation hardening alloy content for some real education.
17-4PH has a measly 0.05% carbon, an incredibly small amount. Like austenitic stainless steels, it has high chromium (17 percent, thus the "17") and four percent of nickel (thus the "4"). Nickel is an austenite stabilizer, and this steel also has a lot of copper (over 4%). Just so you know, copper is detrimental in tool steels but is added here because it helps corrosion resistance (think chemical storage tank, pump body, or piping) and it helps with the whole precipitation process.
At the very maximum, 17-4PH, the hardest precipitation hardening steel has a Rockwell hardness of 40C. This is terribly, horribly soft; not even in the range of tool steels. It's softer than any knife blade, drill bit, sewing needle, spring, or hand saw for wood. This is 12 points softer than the bottom-feeder of stainless steels (420 stainless steel), used in the very worst, cheapest, and lowest grade stainless knife blades from China. Twelve points!
PH steels are not wear resistant in any way, shape, or form, and are only used in low wear resistance applications. The only way their wear resistance can be increased is if they are coated with chromium oxides, titanium nitrides, or other proprietary coatings that will be gone at the first sharpening, should this steel be used for a knife blade. This is why these are not tool steels and not knife blade steels.
This is an example of someone who read about one steel somewhere, but doesn't actually realize that there are many, many types of steels for many types of uses.
"The amount of retained austenite following cryogenic quenching is pretty much negligible (according to all the various papers I've read on the subject). Multiple tempering should not be required for cryo-treated steels, although it should be noted that the cryo-treatment drastically changes the steel's response to tempering, usually increasing the tempering requirement to lower the hardness to any given level. I suspect that precipitation hardening is responsible for the increase of hardness in cryo-treated steels during tempering, and that's not something you can or should try to temper out but rather something you should probably induce in a separate step to make tempering consistent. Cryo-treating a knife twice is more than likely just a huge waste of liquid nitrogen."
My response:
Where do I begin? This guy clearly has no business commenting on anything related to steel, transformation, or treatment. Where in the world do people like this get their information?
For the first sentence, here are some numbers so that you can get an idea of retained austenite amounts depending on quenching process. For most high alloy hypereutectoid stainless steels, these are some averages:
From this, you can see that it clearly matters how a steel is quenched, and the difference is dramatic and profound. I don't know what "papers" this guy has read, but he's woefully ignorant, and the "papers" are clearly wrong.
Multiple tempering is clearly required on all high alloy hypereutectoid steels for the important reasons that I've detailed in tremendous fashion on this very page. The YouTube videos also clearly describe WHY THIS IS REQUIRED BY EVERY STEEL MANUFACTURER, EVERY MACHINIST GUIDE, AND EVERY METALLURGY TEXT OR RESOURCE WRITTEN! Okay, sorry for shouting but really, just making a claim that calls the entire steel industry and all metallurgists, scientists, and machine shops wrong just won't stand.
This guy is stuck on his precipitation hardening (posted in the section just previous to this one) as if he knows what he's talking about. And then tossing out how what is done industrially and by heat treaters as a waste of liquid nitrogen makes it clear to me that he doesn't have the slightest idea of what happens in heat treating. Sad, truly sad.
This is why I pulled the comments from the YouTube channel. You deserve to know the truth, not some ignorant poster's opinion or mistaken belief. Good Grief!

Here's an email about the expense and use of liquid nitrogen:
Jay, you spend a lot on liquid Nitrogen. I am assuming that most methods of reducing that would involve too much capital, e.g. high cost. I was going to ask about a freezer, but I don't see how you would control cooling rate. And a liquid nitrogen maker is surely too high.
My response:
You might be surprised to know how little liquid nitrogen costs, and how little I actually use. I don't have a large, wasteful processor and I'm using mechanical refrigeration as an assist. See the Revco shallow cryogenic freezer above? This helps scavenge heat as well as prolong the use of my liquid nitrogen. I won't get into the technical part of that, but I'm incredibly frugal with my LN usage.
Currently, where I live, liquid nitrogen is about $2.00 per pound, which is extremely economical. I don't pay to store LN, my process does not require it. I've refined the process at this point to where the cost of the electricity to heat treat the blade is more than the quenching and cryogenic aging and equilibrium!
--Jay

Here's an email sent by a knifemaker who has great difficulty in mirror polishing high alloy hypereutectoid stainless steels. The email and my response will make it clear that even the very hippest, coolest, newest and highest alloys have their limited, distinctive, and specific range of use and application. These applications may excel in one area, but may be woefully inadequate in others.
This is why knifemaking is a trade-off, and why some alloys (like the two mentioned below) are best served in thicker, roughly-finished or blasted conditions where high wear resistance takes precedence over corrosion resistance and toughness. This also clearly demonstrates how they should not be used in the kitchen, where blades must be thin, tougher (than other high alloys) and able to resist corrosion. Remember, corrosion starts at the very cutting edge, so this is how important it is.
Hello Jay,
Most of knives I make from stainless PM (powder metallurgy) steels, usually M390 or Elmax, and many of
them are polished. After heat treatment with long cryogenic soak (only -80 degrees Celsius,
but still 36 hours gives decent results) these steels are very hard and have lots of carbides,
and that makes them very hard to grind and polish. Because of workshop limitations and what I
have learned, my procedure is as follows:
I grind with ceramic belts (usually aluminum oxide) to the grit of P600, then hand rub with abrasive paper sheets (only SiC works here and even that hardly) to P2000 and polish with diamond pastes (from 6 to 1 micron). This gives nice bright mirror if done right, but takes great amount of time…that is probably why I am the only one that I know in Poland that is polishing these steels. Is there some other, better way that saves some time? Somehow it is hard for me to imagine that you also sit for hours rubbing the knife with bare hands and abrasive paper.
Do you have any suggestion that could speed up the process? I must note that I have tried going up with grit on the belt grinder, but the only belts with grits finer than P1000 are Trizact from 3M (up to A6/P2500) which just slide over the carbides and leave poor finish, not suitable for further polishing. Moreover, it is not possible to see deeper scratches from earlier steps by grinding in one direction.
This may seem to be basic thing for you, but there are not many people that are polishing knives from which one could learn something. I will be thankful for any advice that will save me some time and help to support my family this way. Thank you for your answer, your good words and your time.
With best regards, B.
My response:
Hello, B.
Thank you again for your kind words. I will do my best to answer your question, based on my own experience and knowledge.
M390 steel made by Bohler (Bohler/Uddeholm) is clearly designed for plastic injection molding dies. Though it’s used also in extrusion tooling, it’s clear that this steel is meant to be used in a high hardness condition (at the higher secondary hardening temper temperature range) with wear resistance being the most sought-after result.
Bohler is not clear on their documentation and this is why these data sheets are so insidious and misleading. The first claim they make is “excellent grindability.” You, yourself have ground the steel; do you think it has “excellent grindability?” I’m guessing that you would claim that it is difficult to grind, and very hard to make a knife out of. Bohler is then making a misleading statement. What are they comparing their claims to? They don’t say, and only give generalities.
Another claim they make is that M390 has “high mirrorfinish polishability.” This, from my own experience is a clear misrepresentation, in actuality, a lie. No steel that has 4 percent of vanadium, is high in carbon and chromium and tungsten will have high mirrorfinish polishability. None! If properly hardened and tempered using cryogenic process, there will be an abundance of extremely hard complex carbides, and they will not polish without tremendous, difficult effort. You have described this yourself. Imagine if you took the steel farther to deep cryogenic processing (which Bohler suggests) and how more abundant the carbides would be, and thus, much more difficult to polish. Bohler is then making another misleading statement.
The third statement Bohler makes is “high toughness.” This is absolutely ludicrous! I work with steel professionals, metallurgists at universities, and they study steels with tremendous instrumentation and equipment and process. They will laugh at you if you try to claim that any hypereutectoid, high alloy tool steel has “high toughness.” All of these steels are tool steels; they are made for wear resistance at the cost of overall toughness. But then, Bohler does not claim what they are comparing the “toughness” to! Perhaps they are comparing it to a ceramic metal or cemented carbide lathe tool insert…
You might think that I’m disparaging Bohler for making claims about their materials, but this is fairly common in the steel trades. I had a similar experience with Crucible; they claimed that their CPMS30V, 90V, and CPMS35VN could be “mirror polished.” When I called them and talked to one of their own metallurgists, he laughed and told me it was a “misprint.” We went on to discuss how data sheets were made in the advertising department, and then we discussed how little of their steels were sold to knifemakers overall. He said that they only liked to sell to knifemakers to “see what they come up with.”
One more thing to consider: these steels are made for extremely high performance at high hardness, and therefore their “peak” in performance will, by necessity, be lowered because, in a knife blade, you must temper them back or they will easily fracture. This same issue exists with knifemakers using high speed steels (often containing cobalt) to make their blades. On the surface, it seems logical that knifemakers want to use the very highest-performing, longest edge-retention steel. So they choose and extreme alloy. However, because it’s a knife, a thin, narrow piece of metal, the knifemaker must, by necessity, temper the steel to a lower overall hardness or the knife owner will easily break it. So the reason to have the high alloy is negated by the fact that the knife must be tempered into a range which prevents brittleness.
Consider that these steels are designed to be used in the condition of HRC 68-70 for their best performance, and a knifemaker will have to temper them back to HRC 58-60 just to prevent brittleness! The whole reason for having the high alloy is negated.
B., the fact that you are taking these steels (Elmax has the same difficulty because of the 3% Vanadium) to a bright polish says more to me about your determination rather than a process procedure.
Here is what I offer: if you must use both of these steels (they are good steels, after all) and insist on mirror polishing them, contact Bohler directly and talk to their engineers and ask them what procedure they recommend to polish their steels. I’m guessing that the metallurgists who prepare test samples will not have the same procedure for polishing as a knifemaker. They typically lap with an automatic machine and finish with diamond paste on velvet, all done by automated lapping. This is not making a knife, but perhaps they may give you some great breakthrough that you can share with all of knifemaking. I’ll be very interested to hear what they tell you.
By the way, the high chromium and high carbon together tend to lower overall corrosion resistance, but Bohler makes no mention of this. This is one of the downfalls of the steel ZDP-189 and you can read about that on my website. Bohler 390 is similar, as it starts losing its temper if you heat it up over the temperature of boiling water! This clearly indicates that it is designed to be used at the point of secondary hardness, tempered at 525°C or 977°F, at which point it loses a tremendous amount of corrosion resistance which is very bad in stainless steel knife blades. Why have a stainless steel blade after all if it lacks corrosion resistance and is brittle?
Now, for the very serious stuff: you are a knifemaker; you need to support your work, business, and ultimately your family on the results of what you do. If your technique for polishing these steels costs you tremendous effort, you need to get paid for your effort. This means that your clients need to recognize the value of the mirror polish on these steels, and they would need to be willing to compensate you for your time. Perhaps they will--only you will know that. If they are not willing to pay large amounts for your extra time and effort, you need to change the way you are finishing this steel, or choose a more reasonable application for the steel, or perhaps a more reasonable steel for your clients and your particular market.
In illustration, I can tell you what I do. Since polishing high vanadium alloys is incredibly expensive and time consuming, and since they will have to be fairly robust and thick (to avoid brittleness), I generally only use them on tactical knives that are media-blasted. I won’t use them on any kitchen knife that has to be corrosion resistant and thin; they are too brittle in thin sections. Steels like ZDP-189, and M390 I probably won’t use for knives, because they lose temper so readily at relatively low temperatures. Read the chart on M390; the highest corrosion resistance exists when tempered between 200°C (392°F) and 300°C (572°F), with the hardness associated at those tempers of 59 to 56.5 HRC. Simply put, this defeats the reason to have the high alloys in the first place!
This is why die steels do not always make great knife steels. I know this is a long email; thank you for your patience!
Sincerely, Jay

He who cannot dance puts the blame on the floor.
--Hindi Proverb
I just had to include this; it pops up continually in knife discussion forums, and makers ask me from time to time about problems they have in heat treat. The funny thing about knifemakers is that, if they don't have the necessary equipment to perform a particular and specific procedure in heat treating, they will often ignore the problems that this creates in their process. Then, they focus on another factor, something they can control. Simply, they ignore the actual issue and blame something else.
They don't do this in a vacuum; there are plenty of other hobbyists and part-time knifemakers who will pile onto the problem, all doing the same thing; ignoring the specific and focusing on something else, tweaking and fudging one or two small, unrelated items when the major issue is simply ignored. Since they don't recognize the inadequacies of the major flaw, it doesn't occur to them that it may be happening in their own work. Perhaps, if I explain it using a recent example, it will make sense—or not make sense—which is why it's a problem in the first place.
A knifemaker has a problem with Nitro V, a fairly unremarkable steel. Assigning the name "Nitro" makes this steel sound high-powered and exciting, but in reality, it's a low alloy, ho-hum stainless steel without outstanding alloy content that would make it difficult to heat treat. It may help to understand that it is of lower alloy content than box cutter blade steel: it's hypoeutectoid (lower carbon), and has no volume of any alloying elements that would make it difficult to work or process in any way. Here's a little more information on Nitro V on my "Blades" page, and why it doesn't even rate for a knife blade, in my experience and opinion.
The blades the knifemaker has created with Nitro V have cracked. This is not a singular occurrence; it's happened repeatedly. He is horrified by this (rightly so) and seeks out information about why this is happening. Other makers have also experienced this with Nitro V, and they conclude that the steel type must be at fault. Still, other makers have not experienced the fault, and the debate starts. The forum participants start discussing the minutia of heat treating, and for some reason, focus on the tempering cycles as an issue. They progress to discussing tempering ovens vs. home ovens and start talking about loading up the home ovens with cast iron pots and pans filled with sand and dirt during tempering, so that the hysteresis (fluctuations in temperature caused by the cycling of the heating device) will be reduced.
They do this because they think that this is something they can control. They totally miss the step between quenching to room temperature, and then quenching to shallow cryogenic temperatures. It's not in the conversation; there is not a thought crossing their minds about why QUENCH CRACKING is occurring in the heat treated steel blades.
Someone hints into the possibility of quench cracking. Quench cracking is well-known and understood in metallurgy, machine shops, and metal fabricator crafts and industries. In fact, if there is any crack in any piece of steel at any time during heat treating, the obvious and instant question that would pop up in any metal shop would be "Why is this quench cracking?"
Nobody ever asks, "Why is this temper cracking?"
Seriously, put the term "Quench Cracking" into any search engine and see the hundreds of thousands of results (all helpful) that come up to understand how prevalent this issue is in metal work. Just for fun, put in the term "Temper Cracking" and you'll find that you are redirected to "Quench Cracking" because you have put in the wrong term—really.
This is a very good indicator of the problem, and at least one poster understands that sudden, drastic changes in steel blade temperature at this time is important. The most drastic part of this is when the knife has already quenched to room temperature, and then it's "put in" the very cold environment of dry ice and kerosene, or dry ice and alcohol, or whatever the hobbyist knifemaker has available. The knifemaker may or may not know about the 4-5 degrees Fahrenheit per minute rule, but since these knifemakers haven't got the equipment or process to actually achieve the 4-5°F per minute procedure, one poster suggests that lowering the blade "slowly" into the cold mush will help. He even says this should take a minute or more.
How does a minute to get to -100°F equate to 4-5°F per minute? Seriously, the cooling of the whole blade needs to occur uniformly throughout the blade, and needs to take a minimum of 20 minutes to get to 100°F below zero. As you touch the blade with the very cold liquid, that area cools suddenly and instantly. One of the main reasons for quench cracking is the temperature differences in the part as it's quenched. The blade must be UNIFORMLY and SLOWLY COOLED at a specific and controlled rate. The crack may occur suddenly, or it may occur later, in tempering, in cycling, or even in use, after the knife is sold!
This is clearly "fudging" the science for the sake of not having the right equipment and process, and somehow, these makers think that this will be successful! This happens only because they have gotten away with it in the past, with other blades, with other steels, and haven't noticed the fault. Maybe they were lucky; maybe the knife owner wonders why his blade wears down so easily or why it's cracked, or why it just isn't all that the custom handmade knife blade steel is supposed to be. The truth is most handmade knives are made this way, and while it's accepted among knifemakers, it should be clear to clients that this is going on.
Would you want a metal shop that makes, say, turbine blades for aircraft engines to be fudging the science for the sake of cheapness? By the way, these cracks can be major and sudden, or microscopic and chronic, and will undoubtedly lead to a poor performing blade or outright failure.
No matter; because they don't have an answer for the 4-5°F per minute rule, they focus instead on the tempering. They discuss this like old ladies swapping recipes, with concern about short-cycling their kitchen ovens, multiple stress relieving cycles, worry about hot spots and air flow during temper, and generally ignoring the root cause of the problem.
How very sad for the person who purchases any knife from any of these guys. This is why I write this, so that if you are a knife-buying client, you deserve to know why your homemade knife might not be up to the task, use, and durability that you would expect after you have paid for it.
You might ask why I don't just jump right into the forum and help these guys. I've tried that before, and here's what happens: they don't have the equipment or logic or experience to learn and change, they don't want to hear that from any "big name" knifemaker, they would rather pile on and cause some conflict or confrontation that increases their post count to 42,000. After all, what does Jay Fisher know about it?
I think it's better to post it here, for all to read who want to know the truth. Some knifemakers will read it and learn, but the important thing is that the knife client, owner, and user reads it and learns who may be fudging the process and why.
Thanks for helping stop wives' tales, myth, fallacies, hyperbole, and ignorance in our tradecraft, science, and art.

Claiming that a steel is a high performer does not make it so.
Look, instead, how the steel is used in the consumer, industrial, and machine tool fields for the truth.
Someone sent me a statement about AEB-L; it's repeated in several venues on the internet. I don't know exactly where the statement originates; this is not clearly revealed or referenced.
Remember this: AEB-L is the very same steel—along with 420 stainless and other equivalents—that has given stainless steel kitchen knives and working knives a bad name. Because of the low carbon, medium chromium content, and because of no other substantial alloying elements, these steels are low performers overall. It's because of these alloys that so many people hate stainless steels to this day, yet knifemakers continue to use them and continue to praise their performance!
As you read this section, please understand that people will try many things to sell steel, particularly steels of lower alloy content. Also understand that if lower alloy content steels were indeed better performers than higher alloy steels, there would be no use for higher alloys and no reason to have expensive, time-consuming, and elaborate heat treatment regimes of high alloy steels. After all, if the lower alloys were the truly superior performers, why bother with the more expensive types at all? Do you seriously think that high alloys are created only to make life more difficult? Don't machinists, metallurgists, and the industrial world know that high alloy steels are not needed? You might be surprised by what is claimed by even so-called experts and even degreed metallurgists.
When I write about steels, it is from two sources: education and experience. While I'm not degreed, I am highly educated and knowledgeable about my field, and actually consult with and speak at universities and with metallurgical specialists in several fields related to my career and profession. I work as a paid consultant in legal cases about metallurgy, and coordinate, evaluate, and advise litigants in steel heat treatment and cutting instrument cases.
If you are a client, patron, knife user, buyer, or knifemaker, it's extremely important to look at the record, the photos, the experience, and the claims of any source of information, including this one that you are reading right now. On this website, you can clearly see who I am, who I make for and how long I've professionally made knives. You are free to judge my reasoning behind what I present, based on my experience. This is demonstrated here on the website by the thousands of knives and many hundreds of testimonials about the knives I've made for everyone from combat soldiers to counterterrorism agents, from professional chefs to collectors of fine art and museums. Unlike people who just share and post forum comments and rudimentary information, I've actually made these knives, in the real world, and applied the science required to do so.
There are many people posting articles, studies, comparisons, and evaluations on the internet, and some of them even have degrees in metallurgy. Most of them do not have the experience of actually making knives, but go on to post borrowed and referenced work by other people: in text charts, graphs, and photographs. Yet they have never made a single knife!
This is a tenuous activity if the website is actively asking for money or donations, even through internet donation programs and fundraising schemes. Using other people's and researcher's works for monetary gain without their consent is not educational; it's a felony federal offense of copyright infringement!
Compare that practice with one you are reading here—open information, for free, for truly educational purposes—and I don't ask for money, donations, or any kind of support, whatever. You can clearly see the difference. As I've written before, I write not because I need to sell knives; I refuse most work and have a years-long waiting period without ever having written a word of what you see here. I'm just saddened when I see a lack of integrity in our tradecraft and I'm determined to bring logic and understanding out of chaos, myth, and hyperbole in this field. This line of work has been good to me, it's been my sole professional career for over 30 years, and this is my honor to give back to knifemaking and knife enthusiasts. People deserve to know the truth.
Some people posting misinformation are knifemakers, but are not making for top organizations or incredibly serious knife users (like military, law enforcement, counterterrorism professionals, or principle security details) yet boast about their knives, performance, and steel details. Some have great followings of groupies, fans, and admirers; some are begging for additional income by fundraising on their web venues.
My point is that no matter who they are, look at their knives. Look at their experience, logic, and craftsmanship. Look at their record. Quoting from other sources is important, but it does not take a knife expert to simply copy and paste studies from outside sources, and then tweak the conversation to influence the reader.
You can see that I, too, use and reference sources for this very page. The difference between me and most other sources is that I've actually made every single knife you see on this website (apart from a handful of collaboratives), and the knives are in the hands of professional users of the very top caliber. It's not about me; it's about the knives and their owners and users. All I ask is that you logically consider the source of all claims, and their own experience, and not simply what they have read in a book, study, or report.
This particular comparison of steel types is of AEB-L, compared to 440C (shock!). AEB-L steel is the same as 13C26; they are both very simple razor blade steels, made by several manufacturers. This steel was originally patented in 1928. It is a very early, basic stainless steel. I contains only 0.67% of carbon, and 13 percent of chromium, making it a low alloy, hypoeutectoid stainless steel. There are no other remarkable alloy contents, but there is a rather high amount of manganese, making the steel easier to work and machine and preserving austenite (yikes!). As stainless steels go, AEB-L barely classifies as stainless, with 12 percent being the classification point. This means that in acidic, corrosive exposures, it will have much lower corrosion resistance than a higher chromium alloy and will rust. Ever see a razor blade rust after being left wet for a long time? Now you understand the limitation of lower chromium stainless steels.
No matter. This steel is simple to work with, and makes a fairly inexpensive knife blade. Lots of guys use it, lots of companies use it. It's used a lot in pattern-welded damascus blades; I've used it myself in this application since it forges and welds easily because it's a rather low alloy steel with low forging and forming temperatures. It is technically a "strip steel," made for rolls and strips and bands, and it is conventionally produced (not a powder metal). It's a good steel for razors, and making cheap, uniform cutting edges. It is not a high alloy; it's a very simple steel. It's easy to grind, easy to forge and form, and easy to sharpen with conventional rocks (novaculite Arkansas stones, water stones, silicon carbide, aluminum oxide, and ceramics). This is because it's not particularly wear-resistant.
The important point is that if AEB-L were far superior to other steels, it would dominate the entire industry of knife blades, and all other steels would be pushed out of existence and manufacture. I've noted before that there is no ultimate, superior steel; all are different and all have their advantages and limitations.
However, it seems that this steel needs to be hype-sold, because these higher alloys are expensive an costly to make a knife out of, and you can't hand-forge them, and you can't make a pretty pattern-welded decor damascus blade out of them without ruining them, but you can with AEB-L.
What is humorous is the rather lengthy explanation that is being used to sell this nearly century-old poor-performing stainless steel. As usual, it's compared to 440C (everything is compared to 440C), and the text below hints at AEB-L as being somehow superior, due to "special" carbides developed. This is bunk, and I'll present the quote in italics and insert my comments so that you can see what is left out of the text and why and how it can be downright misleading.
Claim: "Few know what AEB-L steel is, and those that do, only have heard that it is similar to 440B or 440A."
Reality Check: This starts out badly. Most metallurgists and machinists and metalworkers are familiar with this steel, after all, it was patented in 1928 and it's been used billions of stainless steel razor blades made in the last 75 years. It's actually very similar to 13C26 and 12C27, also low alloy, low carbon, low chromium stainless steels. This steel and its equivalents have been used on millions, perhaps billions, of poor-performing kitchen knives that have given stainless steel a bad reputation overall. Other steel companies make equivalent steels in many countries of the world. Clearly, this comment is directed at those who don't know about steels, the uneducated and inexperienced that will be swayed by what is to follow:
Claim: "The only similarities between AEB-L and 440B or 440A is the amount of carbon."
Reality Check: This is incorrect.
Why would this statement be made? Whoever wrote this has negatively influenced his credibility; every venue that repeats the sentence is spreading misinformation.
Claim: "The fact that AEB-L has only 12.8% chromium by weight compared to the 16-17% in 440A and 440B makes the steels quite different."
Reality Check: No kidding. Lower chromium, lower alloy, less corrosion resistant, tremendously fewer chromium carbides—AEB-L is a lower-performing alloy overall.
Claim: "AEB-L is more similar to a stainless 52100 than 440A..."
Reality Check: This is totally ludicrous.
Claim: "AEB-L naturally forms what is called the K2 carbide, the harder of the two chromium carbides, compared to the K1 carbide, which is formed in steels such as 440C. The K2 carbide is about 79 on the Rockwell C scale, compared to 72 for the K1 carbide. "
Reality Check: What is "naturally formed?" There is no such thing, and now we've descended to the ridiculous. I've mentioned before how the goal is to compare every steel to 440C because 440C is the benchmark for steel comparison. This is because 440C has a long-standing reputation for high performance and value. Here, a claim of "superior" carbides will lead the casual reader to think that somehow, AEB-L is better, forming a "harder" carbide. The writer of this suggests that "K1" carbide is formed in 440C, but doesn't follow up with the comment, "as well as many other carbides and complex carbide types involving molybdenum and chromium as well as iron, and these carbides are more dense, profuse, and abundant in 440C overall."
Let's get this very clear. There are three basic types of chromium carbides usually formed in high martensitic stainless steels. They are:
Please look carefully at the hardness numbers; they are misrepresented in the original claim that is the subject of this section. All three of these carbides are formed in 440C. In addition, 440C has a notable amount of molybdenum, and with the extremely higher chromium content, the tremendously higher carbon content, and the addition of molybdenum, 440C forms profuse carbides of many types, including molybdenum carbides (Mo3C2) and complex combination carbides (chromium-molybdenum-ferrous carbides). When properly hardened and tempered, 440C forms an extremely tough and hard carbide structure, with higher strength overall as well as higher wear resistance, higher corrosion resistance, and a much higher-performing knife blade! By the way, read more about carbide development on this very page at this link.
Claim: "Through proper heat treatment, AEB-L has fine, evenly distributed K2 carbides."
Reality Check: Now this gets a little confusing, so please try to follow along— Where is the author of this getting his information? Look up "K2 carbides" in your search engine. I'll wait here.
Waiting...
Waiting some more...
Welcome back. You have found that there is a company named "K2 Carbides®" and references to "K2" carbides in documents about silicon carbide, which is not a chromium carbide. There are also references to fibrosity in carbides that use a designation "K2". K1 and K2 are constants in formulas, but in none of my metallurgical steel references are the names (K1 and K2) used. This is not how metallurgists typically define carbides. They are defined by the chemical formulas listed above. The only other place I see this type of terminology is on hobbyist knifemaker discussion forums, related right back to this string of text.
Where does this designation: K1 and K2 originate? It comes from the book "Metallurgy For the Non-Metallurgist" by John Verhoeven, written in 2005. On page 130, he states, "The first two pure chromium carbides have been labeled K1 and K2 carbides by German-speaking authors."
This is using the German language (on American English venues) to identify two carbides by a simpler means than their chemical formulas. Evidently, Verhoeven wrote this in his book because the book is directed toward knifemakers, and he didn't want to confuse the simple-minded reader.
I'm kidding, of course, but there are some very serious issues with Verhoeven's book. I'm going to go into a detailed critique of this book, but after studying it thoroughly, and discussing it with other metallurgists and engineers, it's clearly written to sell a very particular type of steel to knifemakers. This steel is compared to 440C, and some of the claims are downright horrendous.
While the book is a great general overview of steels that can be gleaned from any beginning to intermediate-level metallurgy course on steel and iron, When it comes to the stainless steel chapter, Verhoeven goes off the rails. He's constructed a diagram (with promoted software offered for sale) that compares 440C with AEB-L. In this, the general concept is that 440C has too much chromium, and too much carbon, and therefore makes too many carbides at the expense of making martensite. This is utterly ridiculous, and then in further reading, you discover that he's conveniently left out molybdenum as an element. He admits that the "440C" he has plugged into the chart (software for sale by developers), is 440C without the molybdenum.
News flash: that's not 440C! And too many carbides? In doing this, the direction of the book is that toughness is increased in lower alloy steels. Yes, we know that this is generally the case; nothing new here. Let's make knives out of structural steel; it's so soft and tough that in never breaks, only bends! Another news flash: toughness is not the number one desired property in knife blades; it's wear resistance!
There are so many problems with this book that only a detailed review and critique will make this clear that this is not good knife blade information to reference. I'll present a detailed review, but just to whet your appetite, know that in Verhoeven's book:
Detailed critique of this troubling text is in the works...
But back to the carbides, K1 and K2 (by German authors... ahem). Perhaps this is done for the sake of speedy typing, since making all those subscripts is a pain in coding and typesetting, but this hasn't stopped the use of the traditional carbide chemical designations by scientists, metallurgists, and technical writers. Nonetheless, in high chromium steels:
Compared to the incorrect hardness claims in the original claim above, you can see that hardness isn't much of a concern or delineation here, but the "K2" carbide is a bit harder.
Let's go a bit further and quote from Verhoeven's text, and we discover this comment, "The K1 carbide is perhaps the most important carbide in stainless steels as its formation often occurs along grain boundaries and promotes localized corrosion along grain boundaries, a type of corrosion called intergranular corrosion."
So it's about corrosion? And in looking up this corrosion issue, you'll find that it occurs in sigma phase and is a problem in FERRITIC stainless steels with less than 0.2% carbon! What does this have to do with martensitic stainless steels that have been properly hardened and tempered? Nothing, that's what. Ferritic, low carbon stainless steels are not used to make knife blades in any way, any where!
This whole K1-K2 stuff isn't working for me! What about Cr3C2: a Orthorhombic carbide with a Rc hardness of about 80? It's the most durable carbide and it's not even mentioned! What about molybdenum carbides, chromium-molybdenum carbides, eta carbide formation, and complex carbide development as the main reason for increased wear resistance and toughness? Verhoeven has left all that out.
Let's go on—
Claim: "AEB-L lies almost perfectly on what is called the "Carbon Saturation Line", which means that all of the carbides formed are precipitated carbides, not primary carbides like are formed in 440C, and there is more carbon and a similar amount chromium in solution as compared to 440C."
Reality Check: Whoa, there, partner! Remember that above, I mentioned that this "chart" has displayed 440C without the molybdenum? In other words, that's not 440C, yet it's represented as such for the sake of his argument and point! This is typical of carbide-nonsense created to sell a lower alloy steel and for nothing else. Knifemakers claiming their lower alloys are somehow superior are the bane of our industry; as are suppliers of low alloy pattern-welded damascus decor steel blanks, billets, and blades that repeat this carbide-nonsense. Using the phrase "almost perfectly" suggests that there is some perfection approached here, and this is nonsense.
Since this is educational, let's look over this "Carbon Saturation Line" stuff. The term is from an isothermal transformation diagram and delineates the maximum amount of carbon that austenite can dissolve within itself (when heated to its critical transformation temperature). Above that line, much more profuse and abundant amounts of carbides are formed than ferrites. Below that line, much more profuse and abundant amounts of ferrites are formed than carbides. At that line, the amount of carbides is markedly less than above the line! Yes, being at the "Carbon Saturation Line" is not a good thing for formation of carbides overall. AEB-L is below that line at a full percentage point of carbon. 440C is way above and outside that carbon saturation line. This is why 440C is able to create an abundant and tremendous amounts of carbide, and AEB-L is not! This is why 440C is a hypereutectoid stainless steel and why AEB-L is a hypoeutectoid stainless steel.
This is also why stainless steels like AEB-L and the equivalents have given all stainless steels a bad name in knives overall!
Claim: "Primary carbides are very large. So, through a balanced composition, AEB-L has excellent toughness, edge retention, workability, ease of sharpening, and ease of polishing."
Reality Check: The size of the carbide does not play a role in how a knife blade performs. For more on that, please read about carbide particle nonsense on my "Blades" page. This is right up there with "minimal molecule edge radius" and other nonsense microstructure components as if the knife user is sharpening his blade under an electron microscope and using it to cut bacteria in half.
The term "ease of sharpening" is a huge, hot red flag in steel discussion. When steels are easy to sharpen, they are simply and clearly less wear-resistant. Resistance to wear = resistance to sharpening; this is the physics of all cutting edges. Suggesting "excellent edge retention," and "ease of sharpening" completely lacks integrity—this is an oxymoron.
The very same thing can be said of the phrase "ease of polishing," because easy to polish means easy to abrade, thus lower in resistance to wear (and abrasion). This is why professional and institutional wear testing is an abrasion process... ahem. This is why high alloy steels are so difficult to work with and bring to a high polish; they resist abrasion. The polishing process is abrading with finer and finer grits until these grits and abrasive process produces a surface that has an even surface with scratches too small to be seen by the naked eye. Softer, less wear-resistant steels are plainly easier to polish, and that is often why they are used.
Summary: Guys write this stuff because they are trying to hype-sell their knives, and prefer their particular steel type. They think that if they write up their favored steel type, and put a bunch of misleading drivel in the statement, no one will look behind the curtain and see that the wizard is a lonely old man, putting on a show for the benefit of the smoke and mirrors and awe of the terminology. Meanwhile, they are making blades out of the same steel that caused so many to hate stainless steel knife blades. They are making them with wooden handles without fittings, octagonal (Japanese style) uncomfortable handles with bamboo and plastic, rabbeted, stick-tang weak handles, plain flat grinds, etched surfaces that are impossible to clean, and lots and lots of pattern-welded decor damascus created just for the pretty and not performance.
Worse, much, much worse, is that these pattern welded damascus blade are not food contact safe! They are not safe because they are etched and rough-surfaced and hide bacteria and pathogens, and they do not satisfy the NSF § 7.1 Stainless steel requirement, that alloys with less than 16% chromium must be heat treated by a proper post-weld process. Because they are made of two different steels, there can never be a proper post-weld process, because each steel is different!
All of these steels have to be heat treated correctly, and there are very narrow and specific circumstances, conditions, times, temperatures and stages for proper transformation of each individual steel type. The treatment is a narrow, controlled, and specific process, and that process, when properly performed, only applies to one steel type. In pattern-welded damascus blades, there is no fixed, rigid, and reliable treatment regime since you are dealing with at least two different types of steel! So, no heat treatment will yield a reliable, superior condition of the pattern-welded damascus steel; it's impossible! More about that on my Blades page at "What about Damascus Steel; It's All About the Pretty."
This is why pattern-welded steels of multiple layers are not used in any serious tool application anywhere on the planet apart from handmade knives. This is why there are no damascus pattern-welded valve bodies, drive axles, or injection forming dies. There are no pattern-welded metal shears, ball bearings, or milling cutters.
Apart from damascus pattern-welded steels, whoever wrote this doesn't even mention heat treating or cryogenic processing of AEB-L (which is absolutely necessary for complete conversion), and how carbide formation and final steel condition is entirely dependent on the knifemaker's ability and experience in heat treating.
No matter—even the very best treatment regime will not make AEB-L a better knife blade than 440A, 440B, or 440C. It's less wear-resistant, with no molybdenum, with lower carbon and lower carbide amounts, and not as good a steel than many other high alloy steels, no matter how small the atomic sharpening radius at the electron boundary is. Or is it the radius of the smaller quasi-particles of spinons, holons, and obitrons...? After all, how sharp is sharp... really?
Thanks for helping stop hyperbole, myth, fallacies, sales jargon, nonsense, and ignorance in our tradecraft, science, and art.

Someone posted this question on my YouTube channel:
"Where can I find information on chromium carbide fractions per total carbide volume in different steels, ideally including pre/post cryo?"
My response:
Unfortunately, this is not as simple as it seems, since all steels vary tremendously by alloy and specific condition as well as treatment regime. Carbide volume is a highly researched topic, and the most current and valid information is found in research papers by scholars in metallurgy. Most of these studies are available (for a fee) from research gates, often with memberships. This is only reasonable, since it is very expensive and time-consuming to perform metallurgical and chemical analysis. I suggest that you detail the steel type and specific conditions and treatment, and then enter your information in a research gate for the most current applicable information on the particular steel you are interested in.
I want to go on a bit more, since this is too expansive for the YouTube format.
Knifemakers want information, and mostly this is available on the internet, but not always. Good information, trade information, can be very expensive and highly guarded. Take, for instance, the realm of heat treating. If a company or entity invests many thousands of dollars in heat treating and procedure, protocols and systems, analysis and improvements, are they expected to simply give this data away for free to help competitors? Of course not. This is how information can have extreme value in the modern world.
What many knifemakers are seeking is simple recipes: mix "A" with "B" and warm to "C" and then cool to "D" and your done. They don't often want to bother with real research in their own field. Knifemaking is not general metalwork; it's a specific, highly controlled process that has a very narrow field interest.
There are no massive tomes that reveal heat treating and specific carbide amounts, indexed to steel types, geometries, and specific knife shapes for every conceivable steel used in the tradecraft. If a company is making jet aircraft turbine blades for the military, you can be certain that these companies know the exact and specific contents of their metallurgy. They also are not going to furnish their competitors with their manufacturing process and guarded procedures. No such information reference exists in knifemaking. There isn't even a degree or certification in knifemaking, and there probably ought to be, since the safety of the knife user may be based on the knifemaker's ability to properly treat a blade!
Sure, some knifemakers have posted their recipes and procedures, but none of them is doing actual wear testing by abrasive methods, metallographic analysis using scientific methodology. For example, on one knife forum, one particular participant is accepting test pieces furnished by knifemakers to perform a toughness test. He doesn't know how the steel is treated, he doesn't know the exact chemical analysis and supplier of the steel, he doesn't even know when the steel was purchased or from who. He doesn't know how fast the steel was moved into cryo, how the cryo temperature was established and maintained, or even if it was. He just accepts bits of treated steel from makers for a "comparison." And this guy calls himself a knife metallurgist! In the universities I deal with, he would have failed his statistical analysis course at the first test...
My point is that scientific testing is a highly, HIGHLY controlled process. All testing needs to be done with strict control and in an extremely narrow framework of process. Simply put, as many variables as possible must be eliminated to reach a specific conclusion. For example, if steel testing is done in heat treating, the amount of minutes a test piece sits at room temperature before cryogenic cooling takes place can change many characteristics in the steel metallurgy. In handling samples for testing, all of the samples must be treated exactly the same, at the same time, in the same place and exposure, with strict controls of the process. Otherwise, a finding does not have validity. Is the D2 steel tested developing more ferrite and cementite because of holding time at room temperature after initial quenching, or is it developing more ferrite and cementite because of a higher tempering temperature surging because the tempering oven door is opened too frequently?
If you think this can get very complicated very fast, you are right. Consider that most knifemakers think a two rivet-handle is good mechanical practice, and a sheath is simply a troublesome afterthought, and you'll wonder why they are worrying about grain bonding and carbide development in high purity alloys.
The answer is, as always, start with the manufacturer's (or foundry's) information on steel treatment, and if you have the time, money, and scientific interest, start making careful and considered adjustments to their recommended procedures based on performance and what you have learned. Document everything! Don't expect a recipe book to tell you that you need to have a maximum of 12 feet between your quenching apparatus and your freezing apparatus so that you eliminate any chance of the occurrence of quench cracking from the volumetric expansion caused by isothermal transformation of retained austenite into martensite during your stride from one piece of equipment to the next! And keep your strides smooth and fast, yet do not incur any wind from your travels...

People who work with steel can come up with some curious practices. I've heard so many of these that it's a bit ridiculous to admit that somehow, they are, well... interesting.
It reminds me of the old guy I worked with when I was an industrial electrician, who swore that electricity flowed from positive to negative. We got in a heated argument, and he threw out his many decades of experience as proof that he knew what he was talking about. After all, he had been around since Edison. I asked him how it was then, that he couldn't actually fix the DC elevator control circuit we were working on, based on his expansive knowledge. He swore at me, left the jobsite, and I made the repair myself, based on my understanding of how things work. Hint: always think Tesla, not Edison.
Now, I'm laughing, because you could claim I'm one of those old guys who stubbornly insist they know what they are doing, so this hits home. After 40 years of knifemaking, I can stand on my experience to make certain claims, but here's the difference: I'm constantly learning, clarifying, detailing, and presenting what I've learned, and I have quite a few examples of how this applies to the real world. What you see on this website are real knives, each one is a learning experience, each one another step in the understanding of this design, material, and use.
One thing I'm dedicated to is exposing and stopping all the falsehoods and mystical hoopla that surrounds knives, steel, and our tradecraft. In this next email, you can see how people could be fooled, how some of the information is valid, and how ridiculous knife beliefs are.
Please, stop this kind of stuff when you see or hear it, and always try to educate those around you. They'll understand and appreciate it in the end.
Hello Mr. Fisher,
I hope this mail finds you in good health and that the masterpieces are continuing to come out
of your shop and plaster smiles on your client's faces.
I have a question I was hoping I'd get a proper answer from you. Surprised my by how all my Google search results just turned empty or irrelevant results and hence, asking this to you as a learner. Hope you don't mind.
I work in a company that builds special purpose machines for nearly all the major players in the automotive and emissions sectors. We typically work with mild steel, low carbon steel for basic structures and go for steels like EN36 and higher for parts in frequent contact, O1, D2 for tools, dies etc. I have some experienced tool and die makers (>25 years of experience) in my department who usually take a plate, move it around in their hands, feel the weight of the plate and conclude whether it is hardened or not. And I've checked that their observations are always right.
My landlord also works in a similar industry and he taps pieces against each other, listens to the sound and tells whether it is hardened or not. Regarding the sound, I have at least some basic idea that the more 'glassy' it sounds, the harder it is (please correct me if I'm wrong) but I could not get any answers on the change of weight with respect to hardness.
Can you please explain this strange phenomenon? Or is it something basic that I slept through in class? This has been bugging me for quite a while and after many failed Google searches, I'm here hoping to get my mind cleared. If there's information regarding this on your website that I might have missed, please point me in that direction. Thank you very much in advance for your time.
Sincerely,
B.
My response:
Hi, B.
What a great story; I had to laugh a bit at that.
I do understand the old practice of thumping a piece of steel and listening for a ringing sound. Steel crystalline allotropes are entirely different after hardening, and there is a bit of merit there, though not much, since tempering steel would take away some of the the extremely hard, brittle structure that causes steel to ring higher when hardened. However, there is a difference in sound that can be detected, so that’s not entirely off-base. Steel hardness is frequently checked by a dynamic ultrasonic hardness detector, if that answers your question. But the ear is not so accurate…
The weight argument, however, is ridiculous. There are dimensional changes in steel post heat treat, but they are literally microscopic, so that’s not it.
I’m guessing that while someone may think they are judging by weight (which does not change post-heat treat) what they are really doing is looking at the appearance of the part. Steel looks entirely different after heat treating. Areas that are not finished are darker, “muddier” and show signs of oxidation, no matter how pure the heat treating environment is. If the entire part is finished, some people well-versed in steel can tell a difference in the overall granularity and surface appearance, even without the aid of a microscope. They may think they are judging weight, but if you handed them two different pieces while they are blindfolded, their odds would no doubt be the same as random odds.
This is a magician’s feint, and this kind of stuff happens all the time to us, whether we know it or not. We have, after all, five known senses. Perhaps someone one day will claim they can tell the difference between hardened steel and soft steel by the smell.
Don’t laugh; I was at a knife show decades ago, and a guy was going around picking up knives, looking closely at the blades, holding them at an angle in the light. He would then breathe hard and slow on them, fogging them with his breath. He would examine the condensation on the steel with the blade inches away from his eyes.
I asked him what he was doing and he said, “I’m checking for hardness. If a blade is properly hardened, it won’t hold your breath.”
He nodded respectfully; I guess my blades passed his test. After he walked away, I had to pick up my spray cleaner and scrub all the spittle off of the blades… and he never even mentioned how nice the finish was!
By the way, no matter how “hard” a piece of steel is, does anybody ever consider its temper? I’ll bet you’ve never heard anyone ever say, “This steel feels about the right hardness by judging the weight, but it needs another temper cycle with a cryogenic hold to weigh the right temper for use.”
After all, no steel is used in its fully hardened condition without temper unless it’s a precipitation hardening steel or some bainitic steels for railroad rails and ship deck plates. And nobody is weighing them.
If you want to use another sense to test steel, try my “Carbon Steel Taste Test” for chef’s knives for a real eye-opener!
Jay

It's easy to get swept away with all the advanced alloys on the market. Every one of them has limitations and conditions, and no matter what the alloy set and designation, it's the proper heat treating (hardening and tempering) that sets blade performance. The very best steels are frequently, perhaps usually, poorly heat treated in knifemaking.
Hi Mr. Fisher,
I’ve been checking out your website time to time for a bit picking up information and learning more about knives.
I always thought that steels such as Bohler - Uddelholm M390, CPM M4, CPM S110V were easily better than the 440C steel that you tend to use, but realized how crucial the heat treating step was. Fascinating how that can so drastically change the overall outcome of the blade.
I’m thoroughly impressed with all of your knives. The fit and finish matches and exceeds that of any factory knife I’ve seen. Through this website I’ve understood the true worth of custom and handmade knives.
Hopefully I’ll save up my cash to one day buy your knives!
--J. S.
My response:
Hi, J. Thanks so much for your kind words!
There are a lot of very good steels available to custom knifemakers, but all of them have their limitations and correct applications. Many of them—like M390— are hard to find and purchase in the sizes needed for many knife designs, and some of these steels cannot be mirror polished, limiting their overall corrosion resistance. The foundry may claim “high mirror finish polishability,” while at the same time noting that “there is a haze in the finish.” High alloys made for secondary hardening performance (at a very high tempering temperature) lack corrosion resistance when heat treated in this range. Others lack stiffness, and most knife users request a stiff blade unless it’s a fillet or boning knife, which are by necessity ground very thin. And in a boning and fillet knife, corrosion resistance is usually the highest priority!
Many knifemakers try to make up for hasty and inadequate heat treating process by buying and using a more expensive, higher alloy steel. This just doesn’t make sense. A properly treated alloy of any kind will always outperform any steel that is improperly heat treated, and the money spend on the improperly treated blade is just a waste.
The neat thing that I’ve found is that with refined heat treating process, 440C has tremendous wear resistance, strength, toughness, and exceedingly high corrosion resistance. The steel is not expensive; the heat treating is, and few knife providers are willing to invest in advanced processing for these results.
I do work with a dozen different steel types, but 440C is my most requested steel for these reasons. I’ll keep tuning, researching, and improving my techniques with all of my steels, yet some of my clients have told me that from now on, they only want 440C blades treated with my T3 process.
I look forward to making a knife for you one day!
--Jay
I cannot stress how important heat treating is in knifemaking. Everybody is in a big hurry to make knives; very few guys are researching, testing, and improving their heat treating process as part of the field of knifemaking. They are converting toaster ovens to PID control, they are cobbling together heat treating furnaces from found parts and scavenged components. Many of them are "heat treating" in an open forge and tempering by just heating the steel in an open flame! They're shoving blades in dry ice slurry, they're sticking blades in Dewar bottles of liquid nitrogen. They don't have a plan, they are in a hurry, they want to move the process along without logic, research, or result-based decisions about their process.
They get their information from forums, the very worst place to get hobbyist, non-professional information on a highly controlled, precision process. Forums are great, until you realize that they are filled with other hobbyists, even lifelong dabblers in knifemaking that have no actual visual or proven record of making or supplying the very best knives for the most demanding of uses in the military, tactical, or professional fields. And if "old Joe" on the forum claims you can just shove the blade in liquid nitrogen for an hour or two, it must be right, because "old Joe" has 12,000 posts on the forum—and Dizzybob123 can vouch for his work, and everybody knows the "Dizzy" is a great guy and always posts with integrity...
Where is the proof? Where are the knives? Where is the intensive, verified, detailed record of these great works and creations? Where are the references, the logic, and the reasons?
It's hard to take knifemakers seriously when they don't understand the science and results of just one component (heat treating) of knifemaking. For many of these makers, they are continually, obsessively searching for "the right" steel type, the one that will take a lousy heat treating process and result in superior blade. They throw out the alloy letters and numbers with pride, "I only use super steels!"
Then, you find out they don't bother to triple temper (suggested by the Uddeholm sages themselves), because "Old Joe" on the forums says you don't need a triple temper, anyway. They don't even reach full martensitic conversion temperatures, much less soak times for eta carbide formation. After all, they've used the same lousy, inadequate process on other steels, and they've noticed a little bit of improvement by changing the steel type, so there must be something to it—
Maybe they should just buy a factory knife, regrind it, and just let the factory handle the heat treating.
Probably the most significant take-away from this section is that most clients of custom knifemakers are unable to recognize a failed heat treat process, because all they have known in their lives is lousy factory-treated blades (factories cut corners, too). So they think, "Wow, this M4 blade is outstanding!"
Then they pick up a finely treated 440C blade and their mouth drops open as they cut and cut and cut, and cut...How can this be; it's not CPM M4!?
The thing is that all high alloy, hypereutectoid steels can perform better when properly heat treated. Some have finish issues, some have elasticity issues, some have corrosion resistance issues, some have issues with toughness at the thin cutting edge. A superior, accurate, technically proficient heat treating process will improve all of these, on any steel!
The rest is just advertising letters and numbers. Everybody knows the best steel is BR-549.

A comment about heat treating and O1 came in from my Instagram account:
"I was reading your notes (on this page) on sub-zero and cryo of O1, never even knew that was a thing with O1 . . . so much bad information on the web to try to filter out..."
This is the major problem with the modern knifemaking community. The internet is a great source of information, but you do have to have a heavy filter on the source. For instance, I always encourage new knifemakers to never, ever get their information on a forum, even though it's tempting and forums are heavily promoted by search engines. The reason is because they pay for SEO (Search Engine Optimization), and as my 14 year old granddaughter reminds me: "Google runs everything."
It's unfortunate that what started as a vehicle for researchers in colleges—the internet—has become a commercially-driven advertising agency, where money paid to search engine companies is more important than the actual correct information that is researched, documented, and peer-reviewed. Now, even research is sold, requiring a fee to see a reference paper, a college thesis, or a professional study. In its place, people are looking for quick answers.
They want to know, quickly, how to do a heat treat, quickly, and have a good result, quickly. They don't care to dig deep, everyone promises speed and certainty, no one wants to take the time to understand just exactly what is happening. They want a recipe, and the shorter and simpler the details, the better.
They go to forums for this, and there are plenty of anonymous and named hobby-level knifemakers to give them a quick recipe, based on something they learned from another forum member, or maybe even a knifemaker's website. Since videos and forum recipes are short, fast, and easy to understand, they sell well, like dime novels or (for the younger crowd) podcasts thrown together with a looped track of royalty-free music running in the background like a thorn in your ear.
Add to this that steel foundries and manufacturers well-know that the average buyer of a billet or bar of O1 doesn't have the facility or the interest to do an advanced heat treat, so they offer a simple, easy, and fast way to get a generalized heat treat of their steel. It's not excellent, it's not even great, but it's good enough for most jobs, and it only requires a way to heat the steel and quench it. So the standard recipe for O1 is:
That's it! And that will give you a blade that is reasonably hard, and wear resistant, and okay for a hobbyist knife or a basic tool.
Here's the thing: let's hope and pray that you are not making knives just to make a hobbyist tool. If you're going to go to all of the trouble to make a knife by hand, why not make the best possible knife by hand? Why bother making a knife that has ho-hum performance, ho-hum finish, ho-hum handle, or a knife that rates on the same level as a manufactured or kit knife? Why bother?
Is it because you want to prove that you can work at the same level as other hobbyists? Is it to glean clicks and likes on Instagram? Is it to prove that you are just like a manufacturer, who can produce a knife 1000 times faster, but you are down for the struggle? If it is, I suggest you don't make knives at all; because you are simply wasting your time, and life is, after all, short.
But if the reason you are making knives is because you want to create something better, something more advanced, something remarkable, something that not only is nice to look at but excels in actual cutting performance, then you have to up your game. The forum answers won't be adequate, the supplier's quick and easy heat treat won't bring the steel to the pinnacle of performance. Your knowledge of steel will have to go beyond what can be supplied by the common sources.
This is one of the reasons I've created this page. On it, you will see why it's important to bring steels into the cryogenic range during quenching. For instance, all steels that have more than 0.3% carbon have a martensite finish temperature below zero, and O1 has 0.9% carbon! O1 has a martensite finish temperature well into the cryogenic range, but it is typical to only quench to about 120°F and then temper. This is a safety factor to prevent cracking, but that's for complex machined objects, not simple knife blades. Yet, no one considers this, because easy is always better in their minds.
If you do some serious research, you'll find that cryogenically quenched O1 can have a wear resistance increase of over 400%! Why wouldn't you try to offer this to a knife user?
Look, it's okay to perform a simple heat treat on basic knife, just as long as the client knows this, and doesn't pay a premium for some perceived handmade excellence. The issue is that cheap knives are common and no reason to make knives by hand in the first place. That starts with the condition of the steel, controlled by the knifemaker, who is striving for a premium product.
Page Topics
Hello Mr. Fisher,
Thank you for all that you have posted on your website. I am a totally newbie and hobbyist knife maker. I have sold like 4 knives, haha.
However, I have another inquiry. I read so so so many pages on ur site. A lot of good info there!
Here is my question if u have time. Are there any books, you would recommend for me to read on heat treat and cryogenics.
Ones that after all ur reading and study, u would highly recommend, a top 3 or even ur #1 book.
I want to figure out the cryo soak times, the machines, and transitions from the heat treat oven.
--C. S.
I always try to encourage new knifemakers. It's important to welcome people who are seriously interested in knifemaking.
There are many people who want to make knives, but are either stuck in the 19th century practice of hammering low alloy steels, or are trying to make cheap, unfitted, roughly-finished blades handled with a piece of wood, antler, or plastic. I'm always encouraged to discover guys who want to do it right, since they are actually rare in this tradecraft.
You might believe all knifemakers want to make the best knives, but take a good, close look at what most knifemakers are producing. Look at all the blades with poor grind geometry. Look at all of the blades without fittings: no bolsters, fake bolsters, or no guards. Look at all the stick tang (rabbeted tang) knives with a stub of metal shoved in a hexagon of wood. Look at all the two and three-rivet or screwed-on handles, look at the lousy sheaths. As you begin to notice knives, you'll see this same low-effort, low quality type of knife over and over, and this should make you wonder. If the maker doesn't care enough to finish the blade or include bolsters, what kind of heat treating is he performing? Is it like the rest of the knife: poorly made, unfitted, unfinished?
Of course it is. A guy who doesn't bother to finish the steel isn't going to be taking the time and effort to perform a superior heat treat, especially since it's something the buyer of this kind of knife will never see.
When you read the phrase, "I just got these blades back from heat treat," you'll know, for certain, that the maker is either unskilled overall, or doesn't care about the process, since heat treating is the foundation of knifemaking.
The neat thing is that there are makers who are constantly trying to improve their work, and they are not just beginners. It's important that all of these makers get the information they need to understand and excel in the process, and the foundation of the process of knifemaking is in the heat treat. Knives are, after all, cutting tools, and their treatment and resulting microstructure determines every important property they should possess. Otherwise, it's decor damascus time—something you might want to show around and hang on the wall, but a lousy cutting tool.
The first and most important thing I can recommend to guys like C. S. is never, ever get your information on a forum. Forums and discussion boards are filled with misinformation and errors. This is because the people who really are knowledgeable in metallurgy and treatment are not posting there. While a cursory overview of forums seems to offer viable information, this is misleading. Guys who frequent these venues are not reading studies and metallurgy conference papers, they don't reference graduate level textbooks on steel treatments. If they did, they would tell you that there isn't a simple recipe for superior heat treating that can be done in a small garage or shop built in a Tuff Shed®. You won't find machinists sticking a bar of steel into an open flame-filled tunnel of fire, guessing at the temperature by the color, estimating the time for transformation, and shoving the hot steel into a bucket of canola. This is why I say, over and over, that there is no blacksmith in any machine shop, anywhere.
Guys reading this might get tired of reading what good knifemaking is not, but want to know what good knifemaking is. They want to know how to do it right, but without all the learning and studying and interpreting. The first thing to know is that there is no recipe book for proper heat treating. This is because every knifemaker's environment, equipment, and capabilities are different. This is also because steel foundries and suppliers want to sell steel; they want to keep it as simple as possible. That way, no one is discouraged by a complicated process.
For example, if someone told you that in order to take a photograph of a knife, you would need a complete darkroom and lab, and be able to process transparency film, enlarge, and be able to print positives on polyester. This used to be how the best Cibachrome prints were made (and some still are).
Or, you could take a digital photo with your cell phone camera, and post it to your social media account.
Would you start buying up the equipment for the darkroom and lab? Would you be discouraged? Or would you take the easiest route that doesn't cost you a penny?
Steel suppliers know the answer, so they keep heat treatment protocols simple, and this is not the very best treatment. Additionally, a knifemaker can't go to some sourcebook, and come up with a magic recipe that they can heat and cool their steel to that will yield a superior, penultimate solution for heat treating. It's not about a recipe, it's about a process. The process depends on several things:
You can see that I've opened up a can of worms here, but let's just talk about the first one. After all, that is what C. S. is asking about.
The first place to go to for information is this very website, this very page. If you're reading this far, you are more than interested, and you deserve to know more.
The next thing I'll suggest is what is one of the better books listed in the References at the bottom of this page: "Steels: Processing, Structure, and Performance," Second Edition, ASM International, by George Krauss, 2015. It's a serious read into many different kinds of steel, and you'll read it over and again, and learn more every time.
Swinging the pendulum the other way, here's a book I strongly discourage: "Steel Metallurgy for the Non-Metallurgist, "by John Verhoeven, ASM International, 2007. This book is referred to over and again by knifemakers, and that's because it's written for blacksmith knifemakers by someone who only has the input of blacksmith knifemakers (read the preface). While it has some good basic information, it never mentions high alloy steels, doesn't cover cryogenics at all, and is written with the premise that all knifemakers are hammer-wielding craftsmen who can't and don't work with high alloys or hypereutectoid steels. There are many false assumptions in the text, like toughness is the most important property of knife blades (not wear resistance or corrosion resistance) and that the ultimate steel is low alloy, low carbon AEB-L. By the way, AEB-L is one of the steels that has given stainless steel such a bad name. Don't bother with this book, it's misleading and too short, and it won't answer your questions adequately. Just know that this book only mentions one steel (in the entire book!) by name, and one company that's selling it—in the entire text! Advertising 101. And the word "cryogenic" doesn't even appear in the index...
The next helpful study is The Effects of Cryogenic Treatment on Cutting Tools, Satish Kumar, IOP Conference Series: Materials Science and Engineering, 2017. This is a great, simple overview of the benefits of cryogenic treatment, and a concise, clear work by Dr. Kumar and others. It's open access (available to the public for free), and the additional references listed there will give you a window to where these facts come from and how they originate. Just copy and paste the information above in bold into your browser, and you will find it.
Please don't be discouraged by the technical writing and nature of these dissertations, papers, and texts on the process. Steel is not a simple thing and as you read, keep a dictionary (online) open so that you can understand each term, phrase, and word. Also understand that English is not always the first language of the writers; there is a lot of great research going on all over the world on steels.
By learning about the process, you will then be able to direct your efforts to the other parts necessary: the blade geometry, the equipment, and the steel itself. First, though, a foundation of understanding about steel will be your guide. We are all limited or rewarded with the knowledge of our art, science, and skill.
Page Topics
Factory knives are inferior to fine handmade and custom knives.
I have a short, modest video of a small batch of knives warming after a long cryogenic soak, posted on YouTube. It's a tiny clip, simply demonstrating a moment in the warm-up cycle and the energy exchange as blades go from very cold -320°F (-196°C) to room temperature. It's a tiny part of the cryogenic process, and refers back to this very page of information on the cryogenic treatment of knife blades.
Some people feel compelled to comment, to reveal their own understanding (or lack thereof) of knives, blades, and knifemaking. Here's one of them:
...pointless and time consuming procedure, only to justify high dollar prices from a "handmade knifemaker" that produces very small barche of strange shaped knives and sheaths . This procedure was perfected by some german knife brands ( like Zwilling & Henkels in Solingen-Germany) and needs special machinery and technicians to be well executed.
Let's look closely at this sad comment, so that others can learn from it. If we just ignore these people, and don't call them out, we are contributing to the dumbing down of society. It's time that the internet is actually used as an educational medium, and not as an outlet for ignorance and story. I don't expect this guy to ever learn a thing; his mind is made up, no matter who offers any of that troublesome technical jargon.
The first comment (pointless and time consuming) is a sad commentary on mass ignorance. If this person just bothered to read one textbook, just one of the many studies listed below about cryogenic processing and its advantages, he would have never let this comment come from his bully thumbs onto his keypad. It's sad to think that some people today—in our modern times, with advanced steels, tooling, processing, machinery, and understanding of steels—don't think cryogenic processing matters. He is, simply, wrong; cryogenic processing makes a huge difference in the overall condition and performance of steel.
His next comment is an attempt to insult my knifemaking, and by doing so, insults all knifemakers. To accent "handmade knifemaker" could be seen as an attempt to diminish what I, and thousands of other knifemakers do. His use of the word "barche" identifies him as using the Italian language, as does his name, which I will not bother to use, since he is no source of knowledge or information. A barche is a small boat; a batch is a group of things.
He claims that my knives and sheaths are "strange-shaped." Funny, I have over 500 individual knife patterns, and most of them are recognizable forms. Take, for instance, the chef's knives. Nearly every chef's knife design I make comes from traditional, typical, proven designs, some of which have been around for centuries. He must be commenting only on the knives he sees on the video, which are mostly tactical combat and counterterrorism knife blades. Of course, these would be strange-shaped if you wanted to use them in the kitchen. In any case, all of the knife blades that are shown in the video and the thousand or so that are shown on this website are sold and in the hands of happy owners, so somebody likes the designs, even if it's not him.
The next comment is the most telling. He claims that the procedure was "perfected" by German knife brands. This is typical of someone who is gullible to mass production advertising hype. Advertising by manufacturers is NOT the source of the most advanced technical information. If it was, you would see mass produced knives lasting for generations, requiring very little sharpening, having extremely high value, and you do not. By the way, fine handmade chef's knives do last for generations, require very little sharpening, and retain extremely high value, so there is obviously a substantial difference. This guy has been swayed by the names of Zwilling and Henckels, as if they make the very best knives. They do not, not even close. If you are reading this, you deserve to know why, so let's examine this closely.
The name "Zwilling" and the name "Henckels" refers to the same company, not two different manufacturers. They are not separate; the name of the company is actually "Zwilling J. A. Henckels Knives." They have two lines of manufacture. The "Twin" line (showing the two stick figures on their logo) is made in Germany, and all other Henckels knives (using the name or showing the single stick figure) are outsourced to other countries. Zwilling doesn't identify where these knives are made, and this is omitted probably because they don't want everybody knowing that their knives are made in China, Taiwan, Pakistan, India, or other countries. Otherwise, they would proudly proclaim the source for their knives, right? They do identify their manufacturing interests in Spain, Italy, and Belgium, which makes me wonder about the European Union and their influence on marketplace perspective, but that's another topic. They even have a section of their company that makes Japanese-style knives. They also sell cookware, cast iron, flatware, ceramics, and even recipes. This is, simply, a huge manufacturing firm. That alone means they are limited by their manufacturing process.
If you read enough advertising copy, you'll see that the "Twin" Henckels knives are proud of their "patented stainless steel handle," mentioning the patent over and over. First, this is misleading since the handles are not stainless steel, they are mostly plastic. What they have done is patented the shape of the handle. Look it up; just plug the patent number into the US government patent search information and you'll see it pop right up. The patent was filed in the United States Patent Office (why is a German company issued US patents?) in March of 1996. All the patent is for is the shape—it's roughly described in the patent—and they were so proud of the shape that they didn't want anyone to copy it, at least here in the United States, where patents can be defended. However, none of this matters, because the utility patent was only good for 20 years, so it expired in 2016, so anybody can use their handle shape now. Do you wonder now why I don't get utility patents on my knife designs? What a ridiculous waste of time... but this means nothing, it has nothing to do with the blade or cryogenics whatever. It's just something that stands out in their advertising: an out-of-date handle shape design patent they want to proclaim as a beneficial feature. A bit misleading, isn't it?
Zwilling J. A. Henckels does not mention cryogenics anywhere in their advertisements. They mention "ice hardening," which is a weak, vague, and non-specific term , a term invalid in all of the steel research, regulation, and treatment industries. The correct modern terminology can be found on this page. Unless they have access to ice that is -320°F (-196°C), then they are not performing DCT (Deep Cryogenic Treatment).
The questions are: what steel are they using, and how are they processing? You'll be hard-pressed to find this out; evidently they don't like to let their customers know what steel they are using, offering only generalities. They do this on purpose. It's not to protect some secret special 600-year-old information about magical knifemaking mojo. Humanity knows quite a bit about steels, alloys, use, processing, conditions, and performance. I can only conclude that they are not proud of their steel choices. After all, if I, as a singular knifemaker, can identify the exact alloy and processing on every knife, why can't a huge manufacturer who has a dedicated advertising department with perhaps hundreds of workers offer the same information? They leave it out on purpose; I hope you recognize this advertising trick.
It's important to get some perspective here. Zwilling's best knives sell for $99.00 US; that's not for a single knife, that's for a pair of knives. These are mass-produced, no matter how many "skilled artisans" they claim work on the knives in their manufacturing plant. If they were really "skilled artisans," they would quit working for Zwilling, since (from my own experience) a truly skilled artisan can make a lot more than a $99.00 pair of knives! But this, too, is an advertising ploy; just remember the sandwich chain here in the US that claims that each sandwich is made by "skilled artisans," and you'll get the scheme.
Back to the cryogenics. Zwilling has a trademarked name for their "ice-hardening" process, and they call it "Friodur®" It's a heat treating process, that—by their own admission—only goes down to -94°F (-70°C). This is standard shallow cryogenics, not deep cryogenics, and that is the first limitation. This means that it's done cheaply, with mechanical refrigeration, and it also means that the steel type they are using does not form extensive eta carbides and has retained austenite -or- it's an extremely low carbon, low alloy steel. This, by those of us that know the material dictates the process and result, is obvious. They claim this process has 4 steps. Compare that to my deep cryogenic process of 33 steps that takes a week, and you'll see why fine handmade custom knives can cost a lot more.
Henckels and most German knife manufacturers use a steel identified as X50CrMoV15. This sounds impressive, but it's a common alloy. Using the (mainly European) international standard designation makes you think it's extra super special, since our steel identifications in the US are quite simplified without all the digits and numbers. Lets compare:
| Steel Type | Carbon | Chromium | Molybdenum | Vanadium | Manganese |
| X50CrMoV15 | 0.5% | 15.0% | 0.5% | 0.1% (Trace) | 1.0% |
| 440C | 1.25% | 18.0% | 0.5% | 0.0% | 0.45% |
Alright, here we go. I've compared the "Eurosteel" X50CrMoV15 to 440C, simply because that is what I and other knifemakers are using for a lot of chef's knives, and because 440C is the standard that other steels are compared to. This is because 440C has been around a very long time, for good reason, and used to make extremely high wear stainless steel tools, parts, and components. More about 440C on a dedicated page.
The first and most obvious comparison is that the carbon in 440C is over twice as much. 250% of the carbon in the Eurosteel! Carbon is the number one beneficial alloy element in steel. Higher carbon means harder and more abundant martensite, and thus, more abundant carbides throughout. Lower carbon means a softer, less wear-resistant, weaker knife blade overall with dramatically fewer carbides and lower overall strength. This is a striking and revealing disclosure that Henckels probably doesn't want you to know.
440C has 20% more Chromium, an extremely beneficial component of fine steels. High chromium means greater strength, higher corrosion resistance, and a more abundant formation of hard chromium carbides throughout. Lower chromium means lower strength, lower amount of chromium carbides, and a less wear-resistant blade overall.
Both steels have the same amount of molybdenum, which is good, but because 440C has higher chromium and higher carbon, this means more abundant complex carbides can be formed (chromium/molybdenum/iron carbides). This means dramatically greater wear resistance in 440C.
The "German Standard" Eurosteel steel has some vanadium. However, at 0.1%, this is a trace amount, and contributes little to the wear-resistance of the steel at all. Lack of carbon will make formation of vanadium carbides extremely limited in this steel also.
Manganese is much higher in the German steel, and that makes it easier to machine-forge, or hammer out their blades, in other words, softer. Manganese in amounts higher than .06% are there to reduce deformation while hardening, but manganese is not an equivalent replacement for regular beneficial alloying elements (ANSI, ASA).
The German steel is then a low carbon, low alloy hypoeutectoid stainless steel, similar to the poor stainless steels that have given stainless chef's knives a bad name. It's only better than the poor performers AEB-L and 420 stainless because it has some molybdenum and a trace of vanadium. This is not a particularly good alloy, and this may be why Zwilling J. A. Henckels doesn't crow about it, but only in a generalized way.
One more striking fact about this steel. Henckels claims that their blades range from 56 to 58 HRC, a common advertising trick. They state a range of hardness, leading the customer to believing that the steel can somehow be as hard as a typical knife (58 HRC), when it can't and isn't. The BSSA (British Stainless Steel Association) claims that this steel can only achieve a maximum hardness of 56 HRC. So when you see this "range," you can guess that at its best, this is a low hardness knife blade at 56 HRC maximum, which is rather soft for this application of knife, considering the low wear resistance it offers in this steel type.
I had a great opportunity in the studio when a professional chef brought in his Henckels chef knife. He had used it a lot, and it was worn down at the blade from repeated sharpening. The heel was thick and stopped the edge from "finishing" from repeated sharpening. This is a common flaw in the Henckels design; after repeated and frequent sharpening, the blade won't contact the cutting board near the heel—there is a deep depression there—so the knife can't thoroughly complete the cut. In any case, he wanted to know about the hardness, so I set up the Rockwell tester and had him test the blade, twice, just to be sure. Hardness? 56 HRC. This is the maximum hardness possible on this steel, it can't ever be 58, no matter what Henckels claims. Because the steel is a low carbon, low alloy steel, it's never going to last or hold an edge even close to a higher alloy, high carbon stainless tool steel. But, then, there is the price, and my chef will have to buy another and another as he sharpens and wears away the softer blade.
What do you expect from a manufacturer who makes $50.00 knives? Now you know why they have to be continually honed, steeled, and sharpened just to remain usable, and why every chef learns how to whip and wipe the blade on a steel rod, just to be able to cut with it! But hey, there's the price and availability, and the centuries of "reputation," which really helps out in the kitchen, as you describe why you have to constantly sharpen the blade due to the ancient reputation...
Now let's look at the final comment, because this really needs to be clear. The unknowledgeable person writes what little he knows about the cryogenic process, "and needs special machinery and technicians to be well executed." He is the victim of mass-marketing advertising drivel.
If this knife manufacturer truly had a process for their secret steel that was far and away advanced beyond what other manufacturers were creating, they would run all of their competitors out of business. All of their knives would erase the competition's, by performance alone. Do you wonder why every $50 to $100 knife performs about the same? Sure, there is brand loyalty; chefs don't want to change a recognizable knife for another that they are not familiar with. Companies like Zwilling depend on repeat sales and market recognition, and work very hard to foster fandom. Not with facts, but with advertising generalities and tradition. Why are we stuck with traditional, low-performing knives, anyway? Wouldn't it be nice to buy one knife, just once, that would last for generations of use?
All of these details relate to knives that are distinctly low-end. Yes, Henckels makes low-end knives, no matter what you may read or hear from their advertising department and chefs who own them. Manufactured knives are a different field than fine handmade works; this is why a fine handmade knife can cost 10 to 100 times more! Think about it; if Zwilling J. A. Henckels could, they would be making $3000 knives, wouldn't they? They know better. They can't and don't make a knife that good, and they know that their market is in the mass production universe. This is why they are not concerned with individual custom knifemakers and their knives; they are much more concerned with Chinese manufacturers, Japanese and Pakistani manufacturers, and big box retailers and Amazon access and sales. They aren't creative; the aren't leading the field, they aren't innovating by experimentation, method, materials, or process. They are a dependable, predictable manufacturer, working in low end hypoeutectoid, low chromium stainless steels, plastic handles, and volume above quality.
Factories don't use high alloy hypereutectoid stainless tool steels; they use low alloy hypoeutectoid stainless steels. Factories don't use radical and extended heat treating methods; their low alloy, low carbon steels don't require it and it wouldn't help them anyway. They also use plastic handles instead of premium and everlasting materials (like gemstone), they limit or simply eliminate bolsters or fittings, they don't finish the steel surface at all, and they offer a few dozen or so designs, instead of many hundreds. They won't custom make a knife for an individual client or customer, they offer meager or no accessories, but they are priced cheaply.
I hope this helps you understand how and why factory knives are inferior to fine handmade and custom knives, even in the heat treating and cryogenic processing. They truly are different fields, and the only error here is believing that the two can even be in the same range of discussion and comparison.
The truth is most people cannot afford a fine knife, so they are left with simple market choices based on economy. There is nothing wrong with cheap knives, but there is a lot of misleading and incorrect information surrounding knives, limiting the choices and information the public has about knives overall.
The two fields of knives are similar only in the way that a Ferrari is similar to a Ford Fiesta; they are both vehicles with four wheels. When put that way, it should be simple for most people to understand.
Or maybe an ignorant internet bully would go to Ferrari and insist that they don't know what they are doing, making such fine cars, when Ford has perfected the process.
More about Factory Knives vs. Fine Handmade Knives here.
Page Topics
A nice email from someone who was interested in the quenching rates of high alloy, high speed steels and the effect on the resultant microstructure:
Good afternoon sir,
I've been reading up on your page and I have to say, very well done. This is the most comprehensive knife guide/manual/blog/resource I have yet to encounter. Thank you very much for the details and knowledge. I'm just a knife hobbyist; this is not a career for me. Would I like to make it so? Sure, but that means high production to make the money, which I'm not willing to do just yet. One to 4 knives per month, that's my range right now.
I just wanted to run something past you. I've been trying to get my hands on all the outside resources on knife steel, in particular cryogenic treatments. I came across some research dealing with M2 tool steel, specifically with regards to the cooling rate and martensite transformations at isothermal temperatures. I've attached the PDF.
I was always under the impression that it didn't really matter how fast you cooled the steel. This excerpt derailed that notion. What do you think? Is it safe to say that these conditions could also cause the same affects in other high alloy steels? I mostly deal with CPM steels, 3V, S30V, and 154CM. I am looking for the best way to treat these steels, costs be damned. I only care about making the best knives I possibly can, not about how many I can make. Just looking for some feedback when you get the chance.
Thank you and hope all is well sir,
--S.
It's great that S. is starting out right, by doing some research into the very subject he's pursuing. Too many knifemakers just pop into a forum, read some fast and short steps on heat treating, or glean the very simple and condensed guides on the steel data sheets. If you've read this page up to this point, you should be convinced that the very best treatment of knife blade steels cannot be found on those simple sources. Read any research paper and you'll see how much science is behind the allotropic manipulation of steels and the resultant effects. Most people don't want to study to understand the complexity, they just want to make a knife like baking a cake; a recipe, common tools, and a finished piece they can sell.
But, as S. knows, there's much, much more to the process, and hooray for him, for diving deep into the metallurgy.
He sent me an excerpt of a research paper, titled, "Effect of Cooling Rate in Liquid Nitrogen on the Transformation Rate of Retained Austenite in Steel R6M5," by Popandopulo, A. N.; Zhukova, L. T.; Yaroshenko, A. G. This was published in Metal Science and Heat Treatment, Volume 25, Issue 5, pp.334-337 in 1983.
The article is about R6M5 steel, and while not exactly M2, it is equivalent. The R6M5 is a high alloy steel, with lots of molybdenum and tungsten and some chromium, and is technically a tungsten-molybdenum high speed steel. High speed steels are made to cut other metals at a high rate of surface feet per minute, and can withstand high temperatures.
To answer one of his questions, yes, these results can be translated to other high alloy hypereutectoid steels, since most high alloys respond similarly, such as secondary tempering ranges, martensitic transformation, and multiple tempering cycles.
The conclusions of the paper reveal that:
The reason so many knifemakers are stuck on shoving knife blades directly into liquid nitrogen probably originates from the millennia-old practice of shoving low alloy and plain carbon steels into water to quench them. This is fine for low alloys and plain carbon steels, and it's a hard habit to break. The idea that liquid nitrogen is just a very cold water-like liquid, and you just shove the blades in there is wrong, particularly when dealing with high alloy steels. The quenching severity required for transformation is much, much slower in these high alloys, and this older research illustrates some of the reasons why. This is why, in the section above, the 4-5 degrees Fahrenheit per minute rule is so important.
Though he says he doesn't use it, let's look at the steel (M2) just to be clear.
There are a lot in knifemaking that think that if they just use a steel that works for high speed use, it's going to work for a knife blade. How many times do I have to say this? Knives are not high speed tools!
M2 is a good alloy for its use, but these high speed alloys are designed to be treated in the secondary hardening range. This means that they have to be tempered at extremely high temperatures 550°C (1000°F) with the goal of producing a high volume of carbides, usually MC and M6C, dispersed in a matrix of tempered martensite.
To keep this as simple as possible,
Because of lack of toughness, knife blades made of high speed steels must be tempered at the lower range, which negates the entire reason for using a high speed steel in the first place!
This is why I don't recommend these steels for knives. A knife must have a good degree of toughness to prevent fracture. Most blades also should have corrosion resistance, and if applicable, they should be Food Contact Safe. M2 is not Food Contact Safe in any condition, and the benefits of using a high speed steel are not realized in knifemaking, since they can't and shouldn't be hardened in the secondary range in knife applications. See the related article above: Bohler M390 and Elmax finishing problems.
One more thing: M2 is an ugly steel, often with waves of weird texture throughout. So, it has to be left flat, or sanded: not a very appealing steel. Yes, I have made a knife or two with it and understand the limitations.
There are so many other steels that make excellent hand knives that don't need or require secondary range hardening to embrittle them and make them even less corrosion-resistant than they already are so the maker can claim a high hardness.
Page Topics
I received a very nice, polite email from another knifemaker who trying to navigate his way through steels, knife blades, and applications. Because of the detail, and because I know other knifemakers would also like this information, I thought it would be best to answer it here, where it could benefit more readers.
Good morning Jay.
I hope you and your family are doing well in these trying times.
I am, like many, a great fan of your work and site. I would like to ask your opinion on using A’s for choppers.
I make my knives (so far) with 440C and O1 and am slowly expanding my breadth of styles. A (former SWAT) friend has asked for a beast of a chopper/breaching tool and provided a picture for reference. Its a 12” straight, flat chisel tip blade 1/4 “ thick and 2” wide that can be used as a prybar to boot. A flat prybar with an edge is the easiest description.I don’t want to try with 440C because of the grain boundary embrittlement and I think the O1 would be a bit hard/brittle for the intended purpose too. So now I’m looking at the A’s, 2 or 6. D2 is also available here but I haven’t yet worked A’s or D2 and I’m looking for some guidance.
Could I trouble you for a kick in the right direction, pro’s/con’s on A2/6 etc.?
Have a wonderful Christmas and stay safe.
Thanks,
P. S.
My Response:
Hi, P.
Just curious; where are you getting your ideas on grain boundary embrittlement in hypereutectoid steels?
Merry Christmas and Happy New Year,
Jay
His Response:
Hello Jay.
I have a feeling my foot is in my mouth. My understanding of the grain boundary issue comes
from one of my Prof’s at university and a former boss who is a Mech Eng and likes knives.
I’m now hoping that you will tell me that is incorrect, I do like 440C. I don’t typically argue with folks who have that much education in a topic so when I heard that from two well educated people I believed it. Now your question has me thinking ….
I live in Canada, the tool and stainless steels here all come from the U.S.A. Once shipping, dollar exchange and tariffs are applied the prices get awfully expensive so making numerous blades for testing is not on the table for me at this time (mortgage and babies).
I know enough to know that I don’t know enough. If you don’t mind sorting me out I would appreciate it.
Stay safe and have a wonderful Christmas.
P.
At this point, I wrote back to him referring to this very page and section, because I think it's important to post this information for all those who may have the same questions.
The first phrase that popped up for me was "grain boundary embrittlement." If you do some basic research, you'll see right away that grain boundary embrittlement is a highly researched topic- for austenitic stainless steels. 300-series stainless steels that have been welded are the main concern, and the issue deals with high temperature welding and temperature cycling encouraging intergranular corrosion in austenitic stainless steels. This is a concern in the nuclear, shipbuilding, and industrial field where corrosion resistance is paramount in welded and high temperature exposed austenitic stainless steels. This has little to bearing whatever on 440C used for knife blades:
So, let's put that concern to rest; the people he consulted have simply confused the type of steel (austenitic vs. martensitic). Communication errors like this happen all the time.
More importantly is the issue of the knife design. I've written before that a knife blade is not a pry bar, but what if a client wants a pry bar/knife combination, and he insists on it? Let's go down this path of logic a bit to its conclusion.
Why don't we see pry bars with cutting edges? Seriously, if we could offer that, wouldn't it be great to have a pry bar that is thin enough to cut? Let's simplify this a bit. A razor-keen wear-resistant tough shovel would be great!
When I was very young, I worked for a while clearing swamp land, cutting vines and roots and digging and hacking, and I used a shovel, a machete, and a rake. It was hard work. The shovel wouldn't cut the roots, because it dulled right away. I had to dig out around the root, and attack it with the machete. Every time I hit a rock with the machete, it would flatten, roll, and dull the blade. If I had a big knife to do the chopping job, I'm certain that I would have broken the blade the first rock I hit. I tried sharpening the shovel, but it dulled straight away because of the sand and small rocks in the dirt. If i would have tried digging with a knife, I'm sure I would have snapped the blade, because of prying...
I know this sounds simple, but a knife is not a pry bar, and neither is a shovel. What is a pry bar?
Probably the best exposures I've had to pry bars are in the Fire Service and in Archaeology. I was a Level 2 NFPA Rated Fire Officer, and was a volunteer archaeologist for 5 years. The best firefighter prybar is a Halligan tool. There is just about nothing a Halligan tool cannot breach; it's simply the best tool made for entry and ventilation, and rescue, where the hand alone can control the action. What are these made of?
Low alloy carbon steel and low carbon stainless steel.
Why? Because they are thick and heavy and weighty, and are not thin enough to cut. If they were made into a thin cutting edge, they would just bend, like a cheap machete or a shovel. Imagine prying open a car door with a shovel or a machete, you'll ruin both of them. But a Halligan tool has the geometry to get the job done.
In archaeology, I worked in ruins that were large cobbles and small boulders, densely packed in fill. To get these out, I used a mechanic's pry bar, also called a combination bull pin or alignment tool. What was it made of?
Low alloy carbon steel and low carbon stainless steel.
The reasons these steels are used is because they are tough, that is, resistant to breakage and fracture. They may be heat treated, but then they are tempered way back so that they are resistant to fracture, and will bend when stressed into the plastic range and not break. The reason they don't break is because they are very thick. There is no thin place on a pry bar, not when compared to a knife.
What about all the media, television shows, and chopping competitions? Don't knives need to be choppers?
Here's my professional career knifemaker opinion: this is all dramatic hype.
The guy clearing brush in a swamp or jungle is using a machete to chop; it's designed for chopping branches, limbs, and even plastic water bottles, should the need arise.
What about game and animal processing? Is someone actually using a big, honking knife to whack on a hanging pig carcass? What? Isn't that what television knifemaking has become? Go look in a packing plant where they process game, and you'll find no one is using a chopper, they are using extremely thin, sharp knives with accurate control to process animals and meat.
What about military combat and weapons? Take a look at my Counterterrorism Knives Page. You'll see what the guys who really do use knives in close quarters combat are using today. While some of the knives are large, they are not chopping through Godzilla's leg to try to sever an artery. And above all, the knives have thin, sharp cutting edges that are hard and wear-resistant; they insist on this.
The discussion then becomes more about geometry than material. You could make a great pry bar out of 440C, but it would be incredibly expensive, and would have to be very thick, and tempered way back so it wouldn't fracture. Any steel used would need this treatment.
If you ground a knife blade on the instrument, you wouldn't want it to break, so you would either leave it too thick and it wouldn't cut well, or you would temper it back and it would easily bend. Safety of the user should be your ultimate concern, and there really is no way to marry the two ideas. Simply put:
The two fields (knife or pry bar) are so widely apart that any combination of the tool that attempts to marry them will severely limit one or the other. Another way to look at it is:
It sounds like what you are trying to make is an axe. Axes are made of low carbon (hypoeutectoid) plain carbon steels (1040-1060). They are thick and heavy, but won't hold a cutting edge, and have to be constantly sharpened in a convex fashion. You can put an edge on one sharp enough to shave with, but in one chop, the edge is dull and gone.
A knife is simply not a pry bar; no matter what material it's made of. The best steel, the finest heat treatment, and the best finish won't change the fact that geometry is the main design consideration. It's up to the maker to educate his clients as to the limitations of both.
I'm not trying to be critical; as knifemakers we all will face this someday, a client wanting all the attributes possible on his dream knife. So it's really a matter of balance, informing the client what is reasonable in the geometry and design of a cutting instrument. Maybe, the best answer would be to design a sheath, well-made that could accommodate a good, sharp knife, and a small pry bar, too!
For carcass-chopping enthusiasts, I'll add this for the casual media-savvy reader: television is not the source of viable information on actual professional knifemaking. Maybe some day it will be, but not now.
Page TopicsIn the previous topic, I mentioned a knifemaker's concern about grain boundary embrittlement in stainless steels, particularly 440C. He had been told by metallurgists and educators that this was a concern, and listed it as a reason not to use 440C for a prybar-knife blade. Of course, the word "prybar-knife" shouldn't even ever exist in the human language, and in the section, I detailed the reasons why.
I think it's important to go into more detail about the whole grain boundary issue.
For some reason, 440C gets a lot of negative comment from people who have little familiarity with the steel or its use, even those in the trades of engineering, metallurgy, and knifemaking. I write about it extensively on a special page on the website at this link.
My own personal experience with this steel has been superb; it's truly a great steel, but doesn't "act" like other steels. For instance, it takes a lengthy, detailed process to heat treat and cryogenically process this steel, and small changes in that process can give completely different results. It's a fantastic, proven performer, working in some of the most demanding applications in the fields of industry, transportation, energy, nuclear, and aerospace.
The expression grain boundary embrittlement sounds like a big, serious shortcoming for steels, and it's easy to get swept away just by the sound of the phrase. Knifemakers like to discuss grain issues ad nauseum, even while the geometry of the blade, the grind shape and depth, the handle mounting and materials, and poor to no accessories should be their larger concerns. There really is a lot of shoddy work out there, but discussing grain boundaries is something that may distract from that conversation. More frequently, imagined shortcomings may be a way for knifemakers to excuse high alloy, high carbon (hypereutectoid), and high performing stainless steels from their use. These steels are clearly better steels for knives, and because most knifemakers do not "do" them well, they look for excuses. A grain boundary issue is often an excuse, because, after all, who is looking at grain boundary when they buy a knife?
For those of you who do care, and wonder what this is all about, here's some useful information.
300-series steels are austenitic; they are comprised of austenite when at room temperature. This happens because they are low in carbon, high in chromium, and high in nickel. Nickel is an austenite stabilizer, but that's not the whole story. As these austenitic stainless steels are heated in the 1050°C-1150°C range (1922°F-2102°F), they take all the carbon they have into solution. However, when cooled quickly from this temperature range, they will form a supersaturated solution at room temperature. But if they are cooled slowly, the carbon is rejected and forms M23C6 carbide. This carbide (though small in volume) can nucleate at grain boundaries as a particle or even an array of particles. These adversely effect low temperature ductility. This means that the steel is not as ductile (pliable or flexible) at room temperature. Because the M23C6 carbide needs a lot of "M" (referring to a mixture of metal atoms, which, in this case is iron and chromium), it depletes the region immediately surrounding it of chromium. This changes the electrostatic potential to anodic, and it can cause corrosion at the location where the chromium is depleted. This is called "sensitization." Corrosion is prevented in stainless steels because of the formation of chromium oxide on the surface of the steel when exposed to oxygen.
This effect (grain boundary corrosion or sensitization) happens also in ferritic stainless steels, and less often in martensitic stainless steels, and more notably in 12% by weight chromium and lower carbon-bearing martensitic stainless steels.
Whew! Let's tear this apart a bit to understand it and how it might relate to knives.
Even if a knife blade has a welded area, and it has a potential to be sensitized, such as in welding a 300-series stainless steel rod on a 440C tang, it's a simple thing to perform a post-weld process. Here's how it works: Heat the steel to 950°C-1100°C (1740°F-2012°F). This allows the M23C6 to re-dissolve. Air cool. This quenches it rapidly enough so that the nose of the time-temperature-precipitation diagram is missed, and formation of the carbide doesn't happen. Then, several tempers of the junction in the lower range tempers back the 440C so it's somewhat more ductile than the blade. These tempers don't affect the 300-series steels as long as they are not heated up past 1700°F.
Note that I'm only writing about welds on tangs, not any part of the blade! If you heat the blade to 1700°F and then air cool, you are quenching and will form martensite, a totally different process. Also, consider that no part of a knife blade is substantially thick, with a large cross-sectional area, so any time a thinner area is heated, it air-cools rather quickly, and will miss the nose of the time-temperature-precipitation curve for M23C6 formation.
Now, you probably know more about grain boundary embrittlement than you wish to! It's really not a knife blade problem or concern unless you've welded a component on the knife blade, and then a simple post-weld treatment and tempering eliminates it from your night terrors and frightful horrors!
Page TopicsHi Jay,
I will be quick and to the point with this, as I am sure you're very busy. I'm having an argument with a friend and fellow knife maker about cryo in the quench process. He believes there is absolutely zero difference made with cryo in the case of simple carbon steels (10XX), and I have a different opinion that all steels benefit in one way or another. I'm trying to locate some scientific reading to back my stance, and have found a few articles supporting my argument, but my friend is relentless. He states that material testing in a lab is different than real life use and that none of the pro heat treaters cryo the simple 10xx steels, so therefore it cannot make a difference.
I've read your entire website for a few years now and I noticed that you mention several of the high carbon tool steels in the cryo portion, but that still isn't enough evidence for my friend. Can you weigh in on this for me with both your opinion/knowledge and any recommended readings that take a scientific approach to supporting a belief?
Sincerely,
J. R. - beginning knife maker and a big fan!
Not a bad question; I'll see if I can help clarify the issue so that you may have peace with your friend and make your knives with some helpful input.
The answer is that it depends on the actual plain carbon steel designation. You've grouped all plain carbon steels into the family of "10XX" and that's a wide field. For the uninitiated, there are a series of carbon steels that are considered "common," or "low alloy," or "plain carbon steels," or the official designation "carbon steels" by AISI and SAE. These are AISI/SAE classifications of 1010, 1018, 1020 and so on, up to 1095: a whole range of numbers in the 10XX group. The last two numbers designate the amount of carbon in the steel, by percentage. The group starts with 1005 which has 0.05% carbon and goes up 1095 which has 0.95% carbon. None of these plain carbon steels have any significant alloying elements apart from manganese (limited to 1.65% max), silicon (limited to 0.6% max) and copper (limited to 0.6% max).
Now, here's where it gets in the weeds a bit. In the Machinist's Guide, it's claimed that these steels are "most often used without a final heat treatment." Which, for the most part, particularly in the lower carbon percentages, is true. For instance, if you're building a tool frame for a belt grinder, or a using 1018 angle iron for a shelving unit, these are never post-weld or post-machining heat treated. This is NOT knifemaking.
In knifemaking, these carbon steels are often used, and they absolutely must be heat treated. The Machinist Guide describes that these carbon steels can be annealed, normalized, case hardened, quenched, and tempered to improve their performance. And that's what knifemakers do with these steels so they will have improved performance in wear resistance, toughness, and overall durability.
Please review the section above titled: Conversion of Retained Austenite. There, you'll see that carbon steels with more than 0.3% carbon have a martensite finish temperature of below zero Fahrenheit, and eutectoid steels with 0.8% carbon have a martensite finish temperature of -58°F, and this is the physical reality from the textbooks and studies listed below in the references. But It is not the industry standard process to quench below zero or perform cryogenic treatment on carbon steels! So what's this about?
Here's the thing: most knifemakers using these plain carbon steels do not quench to below zero, and very few perform cryogenic treatments of plain carbon steels. In the metalworking and machining trades, these low alloy steels are not considered "worthwhile" to spend the money and time to perform cryogenic treatment. If a company is going to spring for the cost of Deep Cryogenic Treatment (DCT), they're usually going to use a steel that has higher performance characteristics than plain carbon steels.
But that wasn't your question, was it? Do these steels benefit from cryogenic treatment? If you read the resources below and search for some current study information available on research gates on the web, you'll find that these steels absolutely benefit from DCT, with results of considerable increases in hardness and wear resistance due to the presence of secondary carbide precipitates. Additionally, the soaking period at cryogenic temperatures had considerable effect of improvement on the hardness, wear rate, and impact strength of the specimen steels tested.
Your friend is wrong, and you, yourself found study results to back up your point.
But your friend is also right; your friend's statement that professional heat treaters do not perform DCT on their plain carbon steels is also correct. They don't do it because the low cost steel itself and its use does not justify the expense of performing DCT for what could be a minor improvement, not because these steels wouldn't benefit from DCT.
The lesson here is more one of cost/benefit, not the scientific basis of heat treating and cryogenic treatment. In simpler terms, why would I, as a professional knifemaker, apply a sophisticated thermal treatment to a piece of steel that is distinctly low alloy, perhaps hand-forged—which introduces all the debris, contamination, and negative effects of the 19th century hand-forging process—to a knife blade in hopes of a bit of improvement?
Why not, instead, use a high alloy steel that has remarkable performance characteristics even without DCT, but excels in value for my knife client? Maybe we can even make the sophisticated transition to high alloy stainless steels which is a positive move most clients appreciate greatly and where DCT and it's cost is justified.
As knifemakers, this is, hopefully, what we do: think of our client and his needs. The choice is stay with hand-forging lower alloy steels, in which case the cost does not justify DCT, or move on to better high alloy steels where the cost is justified in the long-term value of the blade.
I'll toss a question back at you: what do your customers want, a cheaper blade or a better blade?
I write as a service to my community of knife enthusiasts, people who want to know what I think, people who continually thank me for doing so.
Don't get your information on a forum—any forum; professionals don't post there.
I came across a discussion forum mentioning my name. The forum is made up of people who evidently consider themselves knife "experts," though I don't know exactly what their qualifications are. I did not find one knifemaker posting in the topic, but the aim was clearly to spread some hate and discontent over what you are reading on this very website. While they discussed cutting edges and pulled some of my statements out of context to bolster their points, the reason I'm writing about this here is because of comments they made specifically about cryogenic processing and the claims of improvements over wear resistance which they doubt. (Hint) Don't tell the rest of the metalworking and machining industry that cryogenics doesn't work and that they're wasting their time—
The guy who runs the forum claimed that he wrote me once and invited me to respond, but I didn't answer. He did write me years ago, after doing a hit piece claiming that the only measure of a knife's value was in its ability to cut, and therefore my knives weren't worth what I charged for them. He went on to describe how manufactured knives cut just as well as handmade custom knives, therefore the two were equal in his eyes. Evidently, he's never actually made a knife, at least not one that I could find, and no one who I converse with can find, so one needs to question his authenticity. His insistence on cutting ability being the be-all, end-all in knife value was the basis for my section about comparative logic on my Factory Knives vs. Handmade Knives topic here. Take a look at that and come back for some more straightforward talk.
By the way, please don't tell some of the top military and counterterrorism teams in the world that are using my knives that the ability to cut is the only measure of a knife's value, they're likely to give up their "Ari B'Lilah's" for a scalpel... ahem.
The guy who runs this forum has regularly and repeatedly slammed me and my work over the years, and encouraged and allowed discussions about how my website is wrong, my claims are wrong, and I don't know what I'm talking about. Anonymous posters love to pile on there, after quickly scanning my website for some comment they disagree with, and pulling it out of context to reinforce their point. By the way, not a one of them has one of my knives, not a one has ever stepped up to the plate to buy one, and despite the forum participants suggestions, not a one of them, apart from the forum owner, has ever written me offering a correction or suggestion about what they are disputing on their forum.
These forums are often cliques of high school-mentality losers, gathering together to complain about something they know little. Again, not a one of them is a publicly known, successful, and established knifemaker, metallurgist, machinist, or engineer, and none of them post their professional resume, curriculum vitae, or list of accomplishments in this field, including the forum owner. They anonymously grumble and complain about what I write here.
This is typical cyber-bullying and harassment. Men can be very vindictive, hateful, and cruel, particularly if they don't actually have to face the person they are trying to harass, and this goes on all the time on the internet and social media. They never consider themselves bullies, yet use my (and others') names in their childish cowardice. You might note that I never mention them by name directly; instead I clearly explain why I do what I do and the results. This infuriates a lot of forum posters, since I won't go into the middle of their high school clique and become a victim of their bullying. Not a one of them will ever face me, contact me, or offer any logical response; they are the definition of cowards.
A lot of this comes from the pretense that because they are men, they think they are born with an innate knowledge of knives. Ask any man, and he'll have an opinion about knives, and it often comes from manufactured knives advertisement, movies and media, or from someone in their family or clique. When alternative information is presented to them, the bullying begins. They find like minds—usually on forums—other angry, frustrated cowardly bullies, to agree, and confirm their beliefs. When they act this way, they run off anyone who may actually be a professional, who may actually participate in the tradecraft in an exceptional manner, who may have some worthwhile experience and knowledge on the matter. Then, they cry and complain that "no big-name knifemaker" ever posts on their forum.
For a professional, facts and reality are the root of understanding any technical idea, application, or tool. This knowledge comes initially from textbooks and research; it does not come from a forum. However, textbooks are only the start, the professional must then actually apply this knowledge in the real world. In knives, real world testing is not chopping on water bottles, or hacking a two-by-four, or slicing a pig carcass in a staged video competition. Real world testing is having a counterterrorism professional use the knife, the sheath, and the entire rig in close quarters combat. Real testing is having a United States Marine use the knife in the real world situation of hand-to-hand life or death response. It's having a restaurant chef and owner apply the knife every day to provide for not only himself and his family, but also his patrons and employees.
If you are trying to learn more about this tradecraft, science, and art, my advice from being a professional knifemaker for over 35 years and making knives for some of the most demanding users and owners is this: don't get your information from a forum, any forum. Professionals in the field don't post there.
Forums are mostly full of hobbyists, part-timers, and enthusiasts and there is nothing wrong with that. However, the forums are rife with misinformation, hasty and incorrect process, and outright lies. In heat treating, there is an overwhelming perception that speed, haste, and cheap and common equipment will get the job done correctly, and this is usually wrong. For instance, it might be okay to use a forge and a bucket of oil to quench a piece of plain carbon low alloy steel. It might be okay to heat it in the wife's home oven or a cobbled-together toaster oven to temper it. But if you are trying this with high alloy hypereutectoid (high carbon) stainless steels, this is ruined steel. Guys try to use dry ice in quenching, but ignore the fact that it is not even in the shallow cryogenic range, and they don't control the cooling rate at all. There is no aging, no eta-carbide development, no multiple tempering cycles with cryogenic processing in between. Even though modern textbooks on the subject are clear about this, they ignore it. Then, if someone else tries to do exactly what metallurgy professionals recommend, the bullies pile on, telling them they don't know what they are doing!
There are also people who claim to be professionals posting on some forums. Lots of guys like gleaning data from websites and then "presenting" it to forum junkies. The junkies glom onto this as if it's scripture. Am I wrong for asking, "Where are the hundreds of knives these guys have made? Why aren't they posting on metallurgists' and physics scholarly discussion boards with other professionals? Why aren't they doing studies and posting them for peer review? Why are they on a knife forum with hobbyists, trying to get clicks, and asking for website donations? Why aren't other metallurgists: Ph.D.s, professors, and industry professionals posting there?
Forum bullies try to claim that it's wrong for me to say what I believe on my own website, even though I'm a career-long professional knifemaker. They claim it's to protect younger minds may be swayed into the realms of untruth. They claim that the reason I write what I do is because my site is an advertising vehicle, and nothing else. They claim that everything I write is only to make my knives look good.
So, my freedom of speech about the technical specifics of my lifelong professional career should be controlled by anonymous and ignorant bullies on a forum, is that how it should be?
So, here, now, is the time for some clarification. It is true that I get a bit of criticism. The reason I do is because a few people disagree with what I write. Simple enough, and I can accept that they can choose to deny factual references and experience.
The only reason they even know what I think is because I've taken considerable time to post it here on this web site accessible to everyone who has a browser, for free: the largest, most detailed website of any knifemaker in the world and in history. I do this not because I need to sell my knives. I turn down most of the work people request, because I have so much to do already.
As I mentioned about and in various other locations on the site, I don't need to post any of what I do and explain why I do it. I do this as a service to my community of knife enthusiasts, people who do want to know what I think, people who continually thank me for doing so. These are people who send emails to thank me for what I write, who will never, ever buy a knife from me, people who just want to cut through all the nonsense that they read about knives on forums, bulletin boards, factory knife sites, and other knifemaker's websites. I've posted some of their emails on my Testimonials page; please know as you read these that I'm honored and humbled by their comments and goodwill. Also know that the good emails outnumber the bad by about 300 to one, and I've also posted some of the negative ones on my Funny Emails pages for comparison and a laugh.
The main complaint from the forum bullies I mentioned (specifically about this page) is that I make things up. This is, of course, preposterous. If one of these geniuses simply took the time to read, study and clarify what is represented by the scientists, scholars, and professionals on my "References" list below, they would know exactly where this information comes from. This is not merely my opinion; it is what these professionals have determined and proven by specific, controlled studies. Some of the information comes from published books that one has to buy. They've published these studies, they've published textbooks, and they are the reason I make the claims I do about what they have determined. While some logic and practice is presented here by my own experience, my work pales in comparison to what these men and women have determined, discovered, and detailed in their presentations.
Most of the data is available on the internet, in textbooks, studies, and journals (not from knife forums), and it is knowledge and information that was unheard of thirty years ago. Back then, you would have to travel to college and university libraries to read and learn it, and now, for the first time in human history, much of this information is available to the public! What a great time to read, learn, and grow! What a great time to benefit from the studies, experimentation, and discoveries of others! What a great time to move beyond anonymous claims, wives' tales, old misperceptions, and traditions based in limited scope. What a truly great time to be alive!
Yet... you have to read. You have to take the time, invest the time in yourself, sit down, computer, monitor, screen, or book in your face, in the quiet, with a scientific dictionary at your side, a notepad, and sometimes you have to read the material two or three times to fully understand the scope of these professional works and documentation.
Please know that every single day, I read and study, and this takes discipline. I try to write most days, and this takes discipline. I make knives as a metal smith, machinist, jeweler, lapidary, sculptor, leather worker, artist and professional every single day. That takes discipline. It also takes discipline to clarify what is important to my clients, my readers, and the countless strangers who visit this site every day, people with whom I may never meet, never converse with, never know, but who have the discipline and interest to learn what I try to share.
Simply put, you don't make thousands of knives successfully for decades, have pieces in museums, make for some of the top warriors, law enforcement, military, and counterterrorism professionals in the world, for decades, and have novice, faulty, or incorrect ideas and concepts about your trade and craft. It's not my logic or knowledge alone that has created this, though I know I have contributed to my tradecraft and art. It's the people below in my References list, and it's the people who design, refine, suggest, and use my knives in the field, who are the reason I make the knives I do, the way I do, and make the claims I do. Again, you can read the Testimonials to get an idea of their actual experience.
If you are in disagreement, the resolution is simple. I suggest reading before you post anonymously on a forum, from the very sources detailed below. They are not alone, there are literally thousands of scientists daily sharing their new knowledge and studies on the internet and in publications.
Don't get your piecemeal knowledge on a forum, forums are not frequented by professionals in the field of metallurgy. The real guys in the know aren't comparing factory knife A to factory knife B and claiming that the grain structure is damaged (or improved) by a small change in percentage of one of the elements. Real pros are publishing scientific journals, books, theses, dissertations, and then having them peer-reviewed by other real professionals.
Buy a few books, roll up your sleeves, learn, study, and grow the mind that Almighty God gave you. He has blessed you with this amazing device, he has given you the gift of discernment, logic, and curiosity. You are not born with the truth, you must discern it.
If you wonder where a professional knifemaker publishes, know that you are reading one of the very best free sources right now. If you appreciate the truth here, you are the reason I write. If you disagree, please make sure that you have reasonable considerations based on science and physics, backed up by scholarly resources and not some comment by a forum participant who is not actually accomplished in the field. Do continue your research; no one is stopping you from doing that.
Further, if you truly are gifted in the deep understanding knowledge of knifemaking and knives, the oldest implement in our history, then I implore you to pick up a piece of steel and start making. It's been my experience that there aren't enough really good knifemakers to go around. Then, take plenty of photos, offer detailed descriptions, and build your own profession.
Dear Mr. Fisher,
My first degree is in Physics from University of Athens (4 years), and I got a second one in Mechanical Engineering (5 years) from the
Polytechnic Institute of Athens. Now, these degrees were a long time ago, and I
was never an expert in Materials Science, but I know my
metals, and I sell industrial shredders that have cutting tools. What you write about knife materials and steel is 100% correct, and I
wonder how so many people fall victim to the crap and the hype tooted by a lot of manufacturers and even custom knifemakers. Maybe it’s
the internet. After all, as the recently passed Italian philosopher and writer Umberto Eco once wrote, “Social media gives legions of idiots
the right to speak when they once only spoke at a bar after a glass of wine, without harming the community. Then they were quickly silenced,
but now they have the same right to speak as a Nobel Prize winner. It’s the invasion of the idiots.”
George Papadakis
Athens, Greece



| Main | Purchase | Tactical | Specific Types | Technical | More |
| Home Page | Where's My Knife, Jay? | Current Tactical Knives for Sale | The Awe of the Blade | Knife Patterns | My Photography |
| Website Overview | Current Knives for Sale | Tactical, Combat Knife Portal | Museum Pieces | Knife Pattern Alphabetic List | Photographic Services |
| My Mission | Current Tactical Knives for Sale | All Tactical, Combat Knives | Investment, Collector's Knives | Copyright and Knives | Photographic Images |
| The Finest Knives and You | Current Chef's Knives for Sale | Counterterrorism Knives | Daggers | Knife Anatomy | |
| Featured Knives: Page One | Pre-Order Knives in Progress | Professional, Military Commemoratives | Swords | Custom Knives | |
| Featured Knives: Page Two | USAF Pararescue Knives | Folding Knives | Modern Knifemaking Technology | My Writing | |
| Featured Knives: Page Three | My Knife Prices | USAF Pararescue "PJ- Light" | Chef's Knives | Factory vs. Handmade Knives | First Novel |
| Featured Knives: Older/Early | How To Order | Khukris: Combat, Survival, Art | Food Safety, Kitchen, Chef's Knives | Six Distinctions of Fine Knives | Second Novel |
| Email Jay Fisher | Purchase Finished Knives | Serrations | Hunting Knives | Knife Styles | Knife Book |
| Contact, Locate Jay Fisher | Order Custom Knives | Grip Styles, Hand Sizing | Working Knives | Jay's Internet Stats | |
| FAQs | Knife Sales Policy | Concealed Carry and Knives | Khukris | The 3000th Term | Videos |
| Current, Recent Works, Events | Bank Transfers | Military Knife Care | Skeletonized Knives | Best Knife Information and Learning About Knives | |
| Client's News and Info | Custom Knife Design Fee | The Best Combat Locking Sheath | Serrations | Cities of the Knife | Links |
| Who Is Jay Fisher? | Delivery Times | Knife Sheaths | Knife Maker's Marks | ||
| Testimonials, Letters and Emails | My Shipping Method | Knife Stands and Cases | How to Care for Custom Knives | Site Table of Contents | |
| Top 22 Reasons to Buy | Business of Knifemaking | Tactical Knife Sheath Accessories | Handles, Bolsters, Guards | Knife Making Instruction | |
| My Knifemaking History | Professional Knife Consultant | Loops, Plates, Straps | Knife Handles: Gemstone | Larger Monitors and Knife Photos | |
| What I Do And Don't Do | Belt Loop Extenders-UBLX, EXBLX | Gemstone Alphabetic List | New Materials | ||
| CD ROM Archive | Independent Lamp Accessory-LIMA | Knife Handles: Woods | Knife Shop/Studio, Page 1 | ||
| Publications, Publicity | Universal Main Lamp Holder-HULA | Knife Handles: Horn, Bone, Ivory | Knife Shop/Studio, Page 2 | ||
| My Curriculum Vitae | Sternum Harness | Knife Handles: Manmade Materials | |||
| Funny Letters and Emails, Pg. 1 | Blades and Steels | Sharpeners, Lanyards | Knife Embellishment | ||
| Funny Letters and Emails, Pg. 2 | Blades | Bags, Cases, Duffles, Gear | |||
| Funny Letters and Emails, Pg. 3 | Knife Blade Testing | Modular Sheath Systems | |||
| Funny Letters and Emails, Pg. 4 | 440C: A Love/Hate Affair | PSD Principle Security Detail Sheaths | |||
| Funny Letters and Emails, Pg. 5 | ATS-34: Chrome/Moly Tough | ||||
| Funny Letters and Emails, Pg. 6 | D2: Wear Resistance King | ||||
| Funny Letters and Emails, Pg. 7 | O1: Oil Hardened Blued Beauty | ||||
| The Curious Case of the "Sandia" |
Elasticity, Stiffness, Stress, and Strain in Knife Blades |
||||
| The Sword, the Veil, the Legend |
Heat Treating and Cryogenic Processing of Knife Blade Steels |